Different expression of β subunits of the KCa1.1 channel by invasive and non-invasive human fibroblast-like synoviocytes
- PMID: 27165430
- PMCID: PMC4863321
- DOI: 10.1186/s13075-016-1003-4
Different expression of β subunits of the KCa1.1 channel by invasive and non-invasive human fibroblast-like synoviocytes
Erratum in
-
Erratum to: Different expression of β subunits of the KCa1.1 channel by invasive and non-invasive human fibroblast-like synoviocytes.Arthritis Res Ther. 2016 Jun 1;18(1):122. doi: 10.1186/s13075-016-1024-z. Arthritis Res Ther. 2016. PMID: 27251429 Free PMC article. No abstract available.
Abstract
Background: Fibroblast-like synoviocytes (FLS) in rheumatoid arthritis (RA-FLS) contribute to joint inflammation and damage characteristic of the disease. RA-FLS express KCa1.1 (BK, Slo1, MaxiK, KCNMA1) as their major plasma membrane potassium channel. Blocking KCa1.1 reduces the invasive phenotype of RA-FLS and attenuates disease severity in animal models of RA. This channel has therefore emerged as a promising therapeutic target in RA. However, the pore-forming α subunit of KCa1.1 is widely distributed in the body, and blocking it induces severe side effects, thus limiting its value as a therapeutic target. On the other hand, KCa1.1 channels can also contain different accessory subunits with restricted tissue distribution that regulate channel kinetics and pharmacology. Identification of the regulatory subunits of KCa1.1 expressed by RA-FLS may therefore provide the opportunity for generating a selective target for RA treatment.
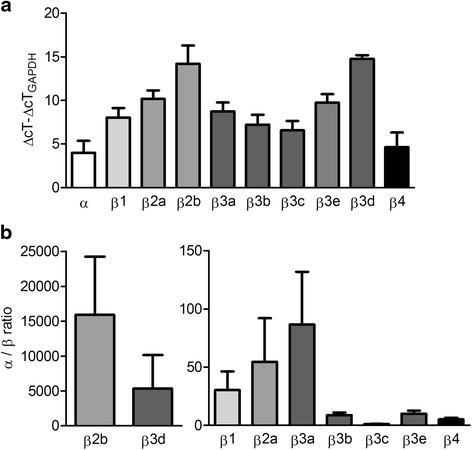
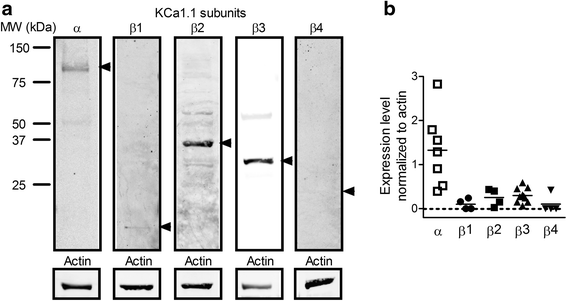
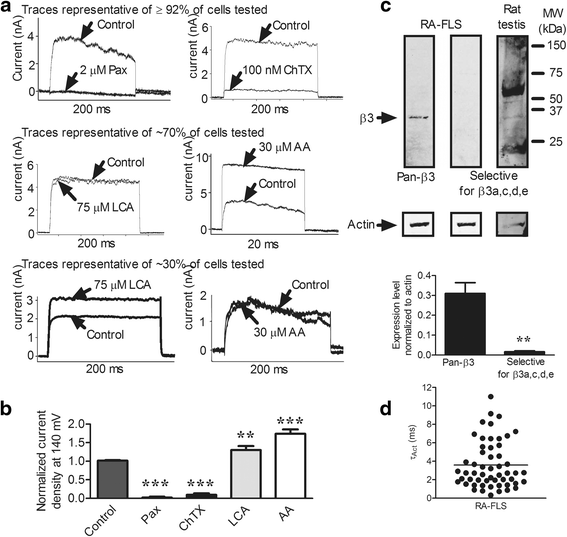
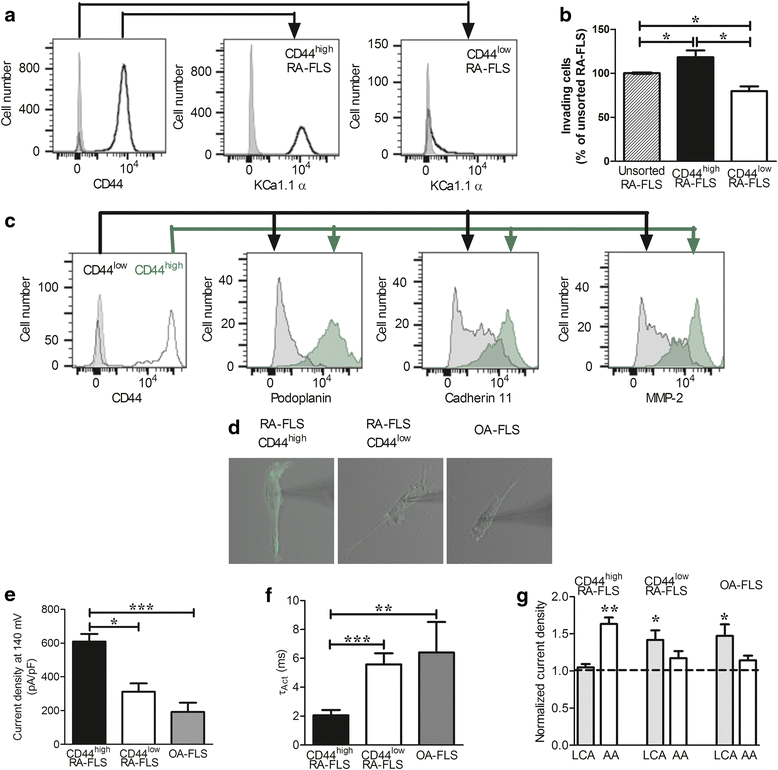
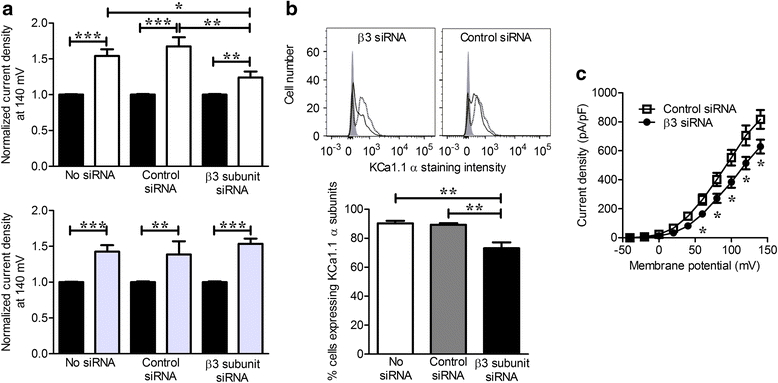
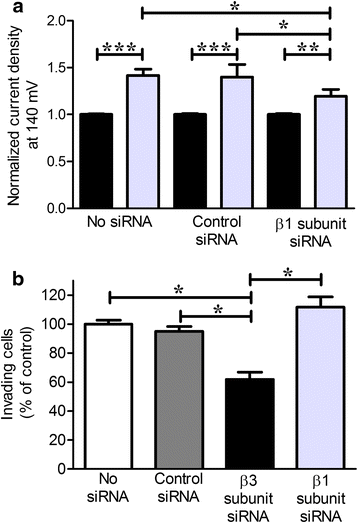
Methods: Highly invasive RA-FLS were isolated from patients with RA, and FLS from patients with osteoarthritis (OA) were used as minimally invasive controls. The β subunit expression by FLS was assessed by quantitative reverse transcription polymerase chain reactions, Western blotting, and patch-clamp electrophysiology combined with pharmacological agents. FLS were sorted by flow cytometry on the basis of their CD44 expression level for comparison of their invasiveness and with their expression of KCa1.1 α and β subunits. β1 and β3 subunit expression was reduced with small interfering RNA (siRNA) to assess their specific role in KCa1.1α expression and function and in FLS invasiveness.
Results: We identified functional β1 and β3b regulatory subunits in RA-FLS. KCa1.1 β3b subunits were expressed by 70 % of the cells and were associated with highly invasive CD44(high) RA-FLS, whereas minimally invasive CD44(low) RA-FLS and OA-FLS expressed either β1 subunit. Furthermore, we found that silencing the β3 but not the β1 subunit with siRNA reduced KCa1.1 channel density at the plasma membrane of RA-FLS and inhibited RA-FLS invasiveness.
Conclusions: Our findings suggest the KCa1.1 channel composed of α and β3b subunits as an attractive target for the therapy of RA.
Keywords: Arthritis; Autoimmune disease; Cell migration; Electrophysiology; Patch clamp; Potassium channel; Regulatory subunit; Synovial fibroblast.
Figures
Similar articles
-
KCa1.1 and Kv1.3 channels regulate the interactions between fibroblast-like synoviocytes and T lymphocytes during rheumatoid arthritis.Arthritis Res Ther. 2019 Jan 7;21(1):6. doi: 10.1186/s13075-018-1783-9. Arthritis Res Ther. 2019. PMID: 30612588 Free PMC article.
-
KCa1.1 channels regulate β1-integrin function and cell adhesion in rheumatoid arthritis fibroblast-like synoviocytes.FASEB J. 2017 Aug;31(8):3309-3320. doi: 10.1096/fj.201601097R. Epub 2017 Apr 20. FASEB J. 2017. PMID: 28428266 Free PMC article.
-
KCa1.1 inhibition attenuates fibroblast-like synoviocyte invasiveness and ameliorates disease in rat models of rheumatoid arthritis.Arthritis Rheumatol. 2015 Jan;67(1):96-106. doi: 10.1002/art.38883. Arthritis Rheumatol. 2015. PMID: 25252152 Free PMC article.
-
Role of glucose metabolism in aggressive phenotype of fibroblast-like synoviocytes: Latest evidence and therapeutic approaches in rheumatoid arthritis.Int Immunopharmacol. 2020 Dec;89(Pt A):107064. doi: 10.1016/j.intimp.2020.107064. Epub 2020 Oct 8. Int Immunopharmacol. 2020. PMID: 33039953 Review.
-
Destructive Roles of Fibroblast-like Synoviocytes in Chronic Inflammation and Joint Damage in Rheumatoid Arthritis.Inflammation. 2021 Apr;44(2):466-479. doi: 10.1007/s10753-020-01371-1. Epub 2020 Oct 28. Inflammation. 2021. PMID: 33113036 Review.
Cited by
-
MicroRNA-192 suppresses cell proliferation and induces apoptosis in human rheumatoid arthritis fibroblast-like synoviocytes by downregulating caveolin 1.Mol Cell Biochem. 2017 Aug;432(1-2):123-130. doi: 10.1007/s11010-017-3003-3. Epub 2017 Mar 20. Mol Cell Biochem. 2017. PMID: 28321538
-
KCa1.1 channels as therapeutic targets for rheumatoid arthritis.Expert Opin Ther Targets. 2017 Dec;21(12):1077-1081. doi: 10.1080/14728222.2017.1398234. Epub 2017 Oct 31. Expert Opin Ther Targets. 2017. PMID: 29076378 Free PMC article. No abstract available.
-
Antioxidant Carbon Nanoparticles Inhibit Fibroblast-Like Synoviocyte Invasiveness and Reduce Disease Severity in a Rat Model of Rheumatoid Arthritis.Antioxidants (Basel). 2020 Oct 16;9(10):1005. doi: 10.3390/antiox9101005. Antioxidants (Basel). 2020. PMID: 33081234 Free PMC article.
-
Targeting KCa1.1 Channels with a Scorpion Venom Peptide for the Therapy of Rat Models of Rheumatoid Arthritis.J Pharmacol Exp Ther. 2018 May;365(2):227-236. doi: 10.1124/jpet.117.245118. Epub 2018 Feb 16. J Pharmacol Exp Ther. 2018. PMID: 29453198 Free PMC article.
-
Regulation of BK Channels by Beta and Gamma Subunits.Annu Rev Physiol. 2019 Feb 10;81:113-137. doi: 10.1146/annurev-physiol-022516-034038. Annu Rev Physiol. 2019. PMID: 30742788 Free PMC article. Review.
References
-
- Gregersen PK, Plenge RM, Gulko PS. Genetics of rheumatoid arthritis. In: Firestein GS, Panayi GS, Wollheim FA, editors. Rheumatoid arthritis. 2. New York: Oxford University Press; 2006. pp. 3–14.
-
- Gulko PS, Winchester RJ. Rheumatoid arthritis. In: Austen KF, Frank MM, Atkinson JP, Cantor H, editors. Samter’s immunologic diseases. 6. Philadelphia: Lippincott Williams & Wilkins; 2001. pp. 427–63.
-
- Gibofsky A. Overview of epidemiology, pathophysiology, and diagnosis of rheumatoid arthritis. Am J Manag Care. 2012;18(13 Suppl):S295–302. - PubMed
-
- Fidder HH, Singendonk MM, van der Have M, Oldenburg B, van Oijen MG. Low rates of adherence for tumor necrosis factor-α inhibitors in Crohn’s disease and rheumatoid arthritis: results of a systematic review. World J Gastroenterol. 2013;19:4344–450. doi: 10.3748/wjg.v19.i27.4344. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

