Blockage of neddylation modification stimulates tumor sphere formation in vitro and stem cell differentiation and wound healing in vivo
- PMID: 27162365
- PMCID: PMC4889367
- DOI: 10.1073/pnas.1522367113
Blockage of neddylation modification stimulates tumor sphere formation in vitro and stem cell differentiation and wound healing in vivo
Abstract
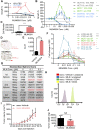
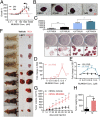
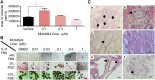
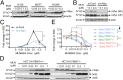
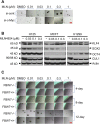
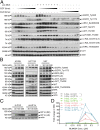
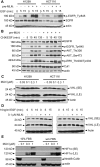
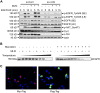
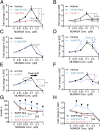
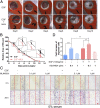
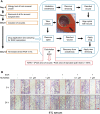
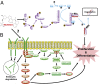
MLN4924, also known as pevonedistat, is the first-in-class inhibitor of NEDD8-activating enzyme, which blocks the entire neddylation modification of proteins. Previous preclinical studies and current clinical trials have been exclusively focused on its anticancer property. Unexpectedly, we show here, to our knowledge for the first time, that MLN4924, when applied at nanomolar concentrations, significantly stimulates in vitro tumor sphere formation and in vivo tumorigenesis and differentiation of human cancer cells and mouse embryonic stem cells. These stimulatory effects are attributable to (i) c-MYC accumulation via blocking its degradation and (ii) continued activation of EGFR (epidermal growth factor receptor) and its downstream pathways, including PI3K/AKT/mammalian target of rapamycin and RAS/RAF/MEK/ERK, via inducing EGFR dimerization. Finally, MLN4924 accelerates EGF-mediated skin wound healing in mouse and stimulates cell migration in an in vitro culture setting. Taking these data together, our study reveals that neddylation modification could regulate stem cell proliferation and differentiation and that a low dose of MLN4924 might have a therapeutic value for stem cell therapy and tissue regeneration.
Keywords: EGFR; MLN4924; neddylation; stem cell; wound healing.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
MLN4924 (Pevonedistat), a protein neddylation inhibitor, suppresses proliferation and migration of human clear cell renal cell carcinoma.Sci Rep. 2017 Jul 17;7(1):5599. doi: 10.1038/s41598-017-06098-y. Sci Rep. 2017. PMID: 28717191 Free PMC article.
-
Neddylation-Independent Activities of MLN4924.Adv Exp Med Biol. 2020;1217:363-372. doi: 10.1007/978-981-15-1025-0_21. Adv Exp Med Biol. 2020. PMID: 31898238 Review.
-
A first-in-class inhibitor, MLN4924 (pevonedistat), induces cell-cycle arrest, senescence, and apoptosis in human renal cell carcinoma by suppressing UBE2M-dependent neddylation modification.Cancer Chemother Pharmacol. 2018 Jun;81(6):1083-1093. doi: 10.1007/s00280-018-3582-z. Epub 2018 Apr 17. Cancer Chemother Pharmacol. 2018. PMID: 29667067
-
Overactivated neddylation pathway as a therapeutic target in lung cancer.J Natl Cancer Inst. 2014 May 22;106(6):dju083. doi: 10.1093/jnci/dju083. Print 2014 Jun. J Natl Cancer Inst. 2014. PMID: 24853380
-
Neddylation Pathway as a Novel Anti-cancer Target: Mechanistic Investigation and Therapeutic Implication.Anticancer Agents Med Chem. 2015;15(9):1127-33. doi: 10.2174/1871520615666150305111257. Anticancer Agents Med Chem. 2015. PMID: 25742093 Review.
Cited by
-
Celecoxib Synergistically Enhances MLN4924-Induced Cytotoxicity and EMT Inhibition Via AKT and ERK Pathways in Human Urothelial Carcinoma.Cell Transplant. 2022 Jan-Dec;31:9636897221077921. doi: 10.1177/09636897221077921. Cell Transplant. 2022. PMID: 35176901 Free PMC article.
-
Neddylation of protein, a new strategy of protein post-translational modification for targeted treatment of central nervous system diseases.Front Neurosci. 2024 Nov 5;18:1467562. doi: 10.3389/fnins.2024.1467562. eCollection 2024. Front Neurosci. 2024. PMID: 39564524 Free PMC article. Review.
-
Coordinating Tissue Regeneration Through Transforming Growth Factor-β Activated Kinase 1 Inactivation and Reactivation.Stem Cells. 2019 Jun;37(6):766-778. doi: 10.1002/stem.2991. Epub 2019 Mar 14. Stem Cells. 2019. PMID: 30786091 Free PMC article.
-
Anticancer drug discovery by targeting cullin neddylation.Acta Pharm Sin B. 2020 May;10(5):746-765. doi: 10.1016/j.apsb.2019.09.005. Epub 2019 Sep 20. Acta Pharm Sin B. 2020. PMID: 32528826 Free PMC article. Review.
-
The Double-Edged Effects of MLN4924: Rethinking Anti-Cancer Drugs Targeting the Neddylation Pathway.Biomolecules. 2024 Jun 21;14(7):738. doi: 10.3390/biom14070738. Biomolecules. 2024. PMID: 39062453 Free PMC article. Review.
References
-
- Hershko A. The ubiquitin system for protein degradation and some of its roles in the control of the cell division cycle. Cell Death Differ. 2005;12(9):1191–1197. - PubMed
-
- Ciechanover A. Intracellular protein degradation: From a vague idea through the lysosome and the ubiquitin-proteasome system and onto human diseases and drug targeting. Bioorg Med Chem. 2013;21(12):3400–3410. - PubMed
-
- Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: Lessons from the first decade. Clin Cancer Res. 2008;14(6):1649–1657. - PubMed
-
- Teicher BA, Ara G, Herbst R, Palombella VJ, Adams J. The proteasome inhibitor PS-341 in cancer therapy. Clin Cancer Res. 1999;5(9):2638–2645. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

