Cancer immunotherapy: the beginning of the end of cancer?
- PMID: 27151159
- PMCID: PMC4858828
- DOI: 10.1186/s12916-016-0623-5
Cancer immunotherapy: the beginning of the end of cancer?
Abstract
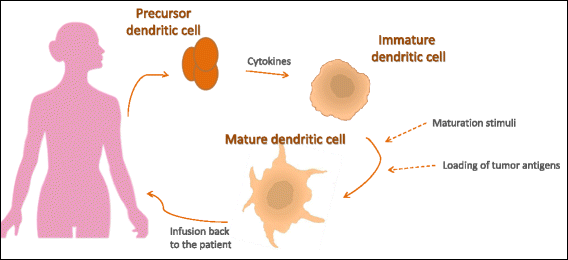
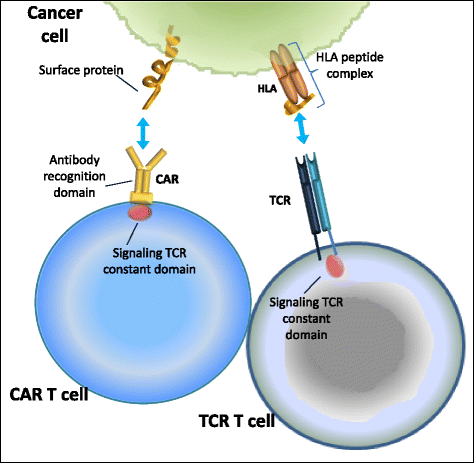
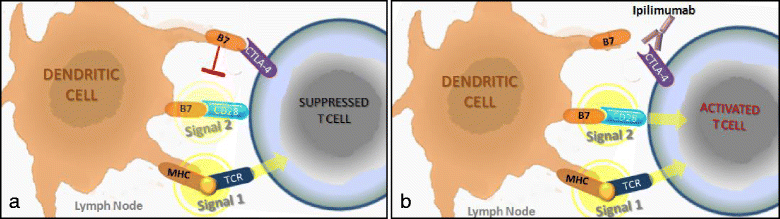
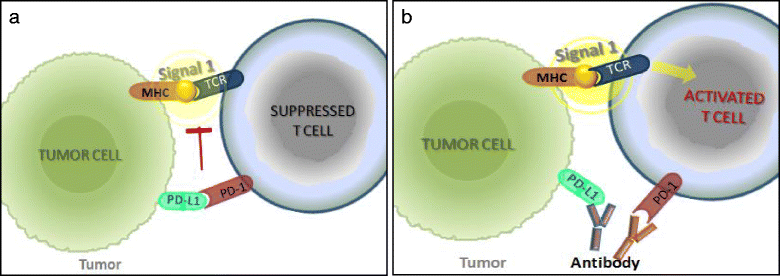
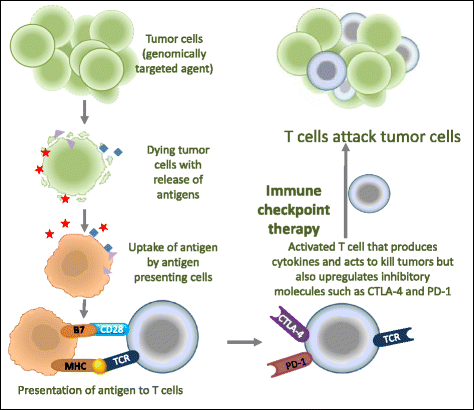
These are exciting times for cancer immunotherapy. After many years of disappointing results, the tide has finally changed and immunotherapy has become a clinically validated treatment for many cancers. Immunotherapeutic strategies include cancer vaccines, oncolytic viruses, adoptive transfer of ex vivo activated T and natural killer cells, and administration of antibodies or recombinant proteins that either costimulate cells or block the so-called immune checkpoint pathways. The recent success of several immunotherapeutic regimes, such as monoclonal antibody blocking of cytotoxic T lymphocyte-associated protein 4 (CTLA-4) and programmed cell death protein 1 (PD1), has boosted the development of this treatment modality, with the consequence that new therapeutic targets and schemes which combine various immunological agents are now being described at a breathtaking pace. In this review, we outline some of the main strategies in cancer immunotherapy (cancer vaccines, adoptive cellular immunotherapy, immune checkpoint blockade, and oncolytic viruses) and discuss the progress in the synergistic design of immune-targeting combination therapies.
Keywords: Adoptive cellular therapy; Cancer; Cytotoxic T lymphocyte-associated protein 4; Immune checkpoint blockade; Immunotherapy; Programmed cell death protein 1; T cells.
Figures
Similar articles
-
Immunotherapy a New Hope for Cancer Treatment: A Review.Pak J Biol Sci. 2018;21(3):135-150. doi: 10.3923/pjbs.2018.135.150. Pak J Biol Sci. 2018. PMID: 30187723 Review.
-
Novel approaches in cancer immunotherapy -- a light at the end of the tunnel.Discov Med. 2016 Jun;21(118):479-87. Discov Med. 2016. PMID: 27448784
-
Immune Checkpoint Blockade in Breast Cancer Therapy.Adv Exp Med Biol. 2017;1026:383-402. doi: 10.1007/978-981-10-6020-5_18. Adv Exp Med Biol. 2017. PMID: 29282694 Review.
-
Immunotherapy for the treatment of breast cancer: checkpoint blockade, cancer vaccines, and future directions in combination immunotherapy.Clin Adv Hematol Oncol. 2016 Nov;14(11):922-933. Clin Adv Hematol Oncol. 2016. PMID: 27930644 Review.
-
Oncolytic viruses as engineering platforms for combination immunotherapy.Nat Rev Cancer. 2018 Jul;18(7):419-432. doi: 10.1038/s41568-018-0009-4. Nat Rev Cancer. 2018. PMID: 29695749 Review.
Cited by
-
A novel inflammatory signature for evaluating immune microenvironment status in soft tissue sarcoma.Front Oncol. 2022 Oct 13;12:990670. doi: 10.3389/fonc.2022.990670. eCollection 2022. Front Oncol. 2022. PMID: 36313634 Free PMC article.
-
Nucleic Acid-Based Approaches for Tumor Therapy.Cells. 2020 Sep 9;9(9):2061. doi: 10.3390/cells9092061. Cells. 2020. PMID: 32917034 Free PMC article. Review.
-
The Contribution of Race to Breast Tumor Microenvironment Composition and Disease Progression.Front Oncol. 2020 Jun 30;10:1022. doi: 10.3389/fonc.2020.01022. eCollection 2020. Front Oncol. 2020. PMID: 32714862 Free PMC article. Review.
-
Chemobrain, Olfactory and Lifestyle Assessment in Onco-Geriatrics: Sex-Mediated Differences between Chemotherapy and Immunotherapy.Brain Sci. 2022 Oct 14;12(10):1390. doi: 10.3390/brainsci12101390. Brain Sci. 2022. PMID: 36291323 Free PMC article.
-
Edaravone: A Novel Possible Drug for Cancer Treatment?Int J Mol Sci. 2024 Jan 29;25(3):1633. doi: 10.3390/ijms25031633. Int J Mol Sci. 2024. PMID: 38338912 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

