Common Genetic Variant Association with Altered HLA Expression, Synergy with Pyrethroid Exposure, and Risk for Parkinson's Disease: An Observational and Case-Control Study
- PMID: 27148593
- PMCID: PMC4853162
- DOI: 10.1038/npjparkd.2015.2
Common Genetic Variant Association with Altered HLA Expression, Synergy with Pyrethroid Exposure, and Risk for Parkinson's Disease: An Observational and Case-Control Study
Abstract
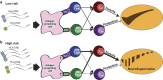
Background/objectives: The common non-coding single nucleotide polymorphism (SNP) rs3129882 in HLA-DRA is associated with risk for idiopathic Parkinson's disease (PD). The location of the SNP in the major histocompatibility complex class II (MHC-II) locus implicates regulation of antigen presentation as a potential mechanism by which immune responses link genetic susceptibility to environmental factors in conferring lifetime risk for PD.
Methods: For immunophenotyping, blood cells from 81 subjects were analyzed by qRT-PCR and flow cytometry. A case-control study was performed on a separate cohort of 962 subjects to determine association of pesticide exposure and the SNP with risk of PD.
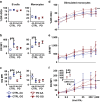
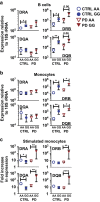
Results: Homozygosity for G at this SNP was associated with heightened baseline expression and inducibility of MHC class II molecules in B cells and monocytes from peripheral blood of healthy controls and PD patients. In addition, exposure to a commonly used class of insecticide, pyrethroids, synergized with the risk conferred by this SNP (OR = 2.48, p = 0.007), thereby identifying a novel gene-environment interaction that promotes risk for PD via alterations in immune responses.
Conclusions: In sum, these novel findings suggest that the MHC-II locus may increase susceptibility to PD through presentation of pathogenic, immunodominant antigens and/or a shift toward a more pro-inflammatory CD4+ T cell response in response to specific environmental exposures, such as pyrethroid exposure through genetic or epigenetic mechanisms that modulate MHC-II gene expression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
Similar articles
-
Impact of human sepsis on CCCTC-binding factor associated monocyte transcriptional response of Major Histocompatibility Complex II components.PLoS One. 2018 Sep 13;13(9):e0204168. doi: 10.1371/journal.pone.0204168. eCollection 2018. PLoS One. 2018. PMID: 30212590 Free PMC article.
-
HLA-DRA is associated with Parkinson's disease in Iranian population.Int J Immunogenet. 2014 Dec;41(6):508-11. doi: 10.1111/iji.12151. Epub 2014 Oct 15. Int J Immunogenet. 2014. PMID: 25319953
-
Association analysis of HLA-DRA in Chinese patients with sporadic Parkinson's disease.Int J Physiol Pathophysiol Pharmacol. 2015 Dec 25;7(4):185-94. eCollection 2015. Int J Physiol Pathophysiol Pharmacol. 2015. PMID: 27073595 Free PMC article.
-
Autoimmunity in Parkinson's Disease: The Role of α-Synuclein-Specific T Cells.Front Immunol. 2019 Feb 25;10:303. doi: 10.3389/fimmu.2019.00303. eCollection 2019. Front Immunol. 2019. PMID: 30858851 Free PMC article. Review.
-
Comparative study of the role of professional versus semiprofessional or nonprofessional antigen presenting cells in the rejection of vascularized organ allografts.Transpl Immunol. 1995 Dec;3(4):273-89. doi: 10.1016/0966-3274(95)80013-1. Transpl Immunol. 1995. PMID: 8665146 Review.
Cited by
-
Inflammation and immune dysfunction in Parkinson disease.Nat Rev Immunol. 2022 Nov;22(11):657-673. doi: 10.1038/s41577-022-00684-6. Epub 2022 Mar 4. Nat Rev Immunol. 2022. PMID: 35246670 Free PMC article. Review.
-
Data-Driven Characterization of Genetic Variability in Disease Pathways and Pesticide-Induced Nervous System Disease in the United States Population.Environ Health Perspect. 2024 May;132(5):57003. doi: 10.1289/EHP14108. Epub 2024 May 16. Environ Health Perspect. 2024. PMID: 38752992 Free PMC article.
-
Quality Over Quantity: Advantages of Using Alpha-Synuclein Preformed Fibril Triggered Synucleinopathy to Model Idiopathic Parkinson's Disease.Front Neurosci. 2018 Sep 4;12:621. doi: 10.3389/fnins.2018.00621. eCollection 2018. Front Neurosci. 2018. PMID: 30233303 Free PMC article.
-
Pesticides and the Microbiome-Gut-Brain Axis: Convergent Pathways in the Pathogenesis of Parkinson's Disease.J Parkinsons Dis. 2023;13(7):1079-1106. doi: 10.3233/JPD-230206. J Parkinsons Dis. 2023. PMID: 37927277 Free PMC article. Review.
-
Whole-genome sequencing to identify rare variants in East Asian patients with dementia with Lewy bodies.NPJ Aging. 2024 Nov 21;10(1):52. doi: 10.1038/s41514-024-00180-2. NPJ Aging. 2024. PMID: 39572598 Free PMC article.
References
-
- Factor SA, Weiner WJ. Parkinson's Disease: Diagnosis and Clinical Management, 2nd edn. Demos Medical Publishing: New York, USA, 2007.
-
- Barnum CJ, Tansey MG. Neuroinflammation and non-motor symptoms: the dark passenger of Parkinson's disease? Curr Neurol Neurosci Rep 2012; 12: 350–358. - PubMed
-
- Bower JH, Maraganore DM, Peterson BJ, McDonnell SK, Ahlskog JE, Rocca WA. Head trauma preceding PD: a case-control study. Neurology 2003; 60: 1610–1615. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

