Spatial and Functional Aspects of ER-Golgi Rabs and Tethers
- PMID: 27148530
- PMCID: PMC4834429
- DOI: 10.3389/fcell.2016.00028
Spatial and Functional Aspects of ER-Golgi Rabs and Tethers
Abstract
Two conserved Rab GTPases, Rab1 and Rab2, play important roles in biosynthetic-secretory trafficking between the endoplasmic reticulum (ER) and the Golgi apparatus in mammalian cells. Both are expressed as two isoforms that regulate anterograde transport via the intermediate compartment (IC) to the Golgi, but are also required for transport in the retrograde direction. Moreover, Rab1 has been implicated in the formation of autophagosomes. Rab1 and Rab2 have numerous effectors or partners that function in membrane tethering, but also have other roles. These include the coiled-coil proteins p115, GM130, giantin, golgin-84, and GMAP-210, as well as the multisubunit COG (conserved oligomeric Golgi) and TRAPP (transport protein particle) tethering complexes. TRAPP also acts as the GTP exchange factor (GEF) in the activation of Rab1. According to the traditional view of the IC elements as motile, transient structures, the functions of the Rabs could take place at the two ends of the ER-Golgi itinerary, i.e., at ER exit sites (ERES) and/or cis-Golgi. However, there is considerable evidence for their specific association with the IC, including its recently identified pericentrosomal domain (pcIC), where many of the effectors turn out to be present, thus being able to exert their functions at the pre-Golgi level. The IC localization of these proteins is of particular interest based on the imaging of Rab1 dynamics, indicating that the IC is a stable organelle that bidirectionally communicates with the ER and Golgi, and is functionally linked to the endosomal system via the pcIC.
Keywords: ER-Golgi transport; Golgi apparatus; Rab1; Rab2; endocytic recycling compartment; golgins; pre-Golgi intermediate compartment; tethering factors.
Figures
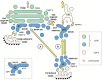
Similar articles
-
Rab1 defines a novel pathway connecting the pre-Golgi intermediate compartment with the cell periphery.Mol Biol Cell. 2006 Apr;17(4):1514-26. doi: 10.1091/mbc.e05-08-0792. Epub 2006 Jan 18. Mol Biol Cell. 2006. PMID: 16421253 Free PMC article.
-
Rab proteins of the endoplasmic reticulum: functions and interactors.Biochem Soc Trans. 2012 Dec 1;40(6):1426-32. doi: 10.1042/BST20120158. Biochem Soc Trans. 2012. PMID: 23176493 Review.
-
The golgin GMAP-210 is required for efficient membrane trafficking in the early secretory pathway.J Cell Sci. 2015 Apr 15;128(8):1595-606. doi: 10.1242/jcs.166710. Epub 2015 Feb 25. J Cell Sci. 2015. PMID: 25717001 Free PMC article.
-
Coordination of golgin tethering and SNARE assembly: GM130 binds syntaxin 5 in a p115-regulated manner.J Biol Chem. 2008 Mar 14;283(11):6957-67. doi: 10.1074/jbc.M708401200. Epub 2007 Dec 31. J Biol Chem. 2008. PMID: 18167358
-
Assembly and Cellular Exit of Coronaviruses: Hijacking an Unconventional Secretory Pathway from the Pre-Golgi Intermediate Compartment via the Golgi Ribbon to the Extracellular Space.Cells. 2021 Feb 26;10(3):503. doi: 10.3390/cells10030503. Cells. 2021. PMID: 33652973 Free PMC article. Review.
Cited by
-
Subcellular Distribution of BALF2 and the Role of Rab1 in the Formation of Epstein-Barr Virus Cytoplasmic Assembly Compartment and Virion Release.Microbiol Spectr. 2023 Feb 14;11(1):e0436922. doi: 10.1128/spectrum.04369-22. Epub 2023 Jan 5. Microbiol Spectr. 2023. PMID: 36602343 Free PMC article.
-
A dual role of ERGIC-localized Rabs in TMED10-mediated unconventional protein secretion.Nat Cell Biol. 2024 Jul;26(7):1077-1092. doi: 10.1038/s41556-024-01445-4. Epub 2024 Jun 26. Nat Cell Biol. 2024. PMID: 38926505
-
Therapeutic Targeting of Rab GTPases: Relevance for Alzheimer's Disease.Biomedicines. 2022 May 16;10(5):1141. doi: 10.3390/biomedicines10051141. Biomedicines. 2022. PMID: 35625878 Free PMC article. Review.
-
Focus on the Small GTPase Rab1: A Key Player in the Pathogenesis of Parkinson's Disease.Int J Mol Sci. 2021 Nov 8;22(21):12087. doi: 10.3390/ijms222112087. Int J Mol Sci. 2021. PMID: 34769517 Free PMC article. Review.
-
RAB2 regulates the formation of autophagosome and autolysosome in mammalian cells.Autophagy. 2019 Oct;15(10):1774-1786. doi: 10.1080/15548627.2019.1596478. Epub 2019 Apr 6. Autophagy. 2019. PMID: 30957628 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

