Inhibitor Discovery for the Human GLUT1 from Homology Modeling and Virtual Screening
- PMID: 27128978
- PMCID: PMC5356226
- DOI: 10.1021/acschembio.6b00304
Inhibitor Discovery for the Human GLUT1 from Homology Modeling and Virtual Screening
Abstract
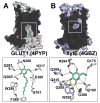
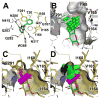
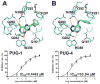
The human Glucose Transporter 1 (hGLUT1 or SLC2A1) is a facilitative membrane transporter found in the liver, intestines, kidney, and brain, where it transports sugars such as d-glucose and d-galactose. Genetic variations in hGLUT1 are associated with a broad range of diseases and metabolic disorders. For example, hGLUT1 is upregulated in various cancer types (e.g., breast carcinoma) to support the increased anaerobic glycolysis and the Warburg effect. Thus, hGLUT1 is an emerging therapeutic target, which also transports commonly used cancer biomarkers (e.g., (18)F-DG). In this study, we use computational prediction followed by experimental testing, to characterize hGLUT1. We construct homology models of hGLUT1 in a partially occluded outward open ("occluded") conformation based on the X-ray structure of the E. coli xylose transporter, XylE. Comparison of the binding site of the occluded models to experimentally determined hGLUT structures revealed a hydrophobic pocket adjacent to the sugar-binding site, which was tested experimentally via site-directed mutagenesis. Virtual screening of various libraries of purchasable compounds against the occluded models, followed by experimental testing with cellular assays revealed seven previously unknown hGLUT1 ligands with IC50 values ranging from 0.45 μM to 59 μM. These ligands represent three unique chemotypes that are chemically different from any other known hGLUT1 ligands. The newly characterized hydrophobic pocket can potentially be utilized by the new ligands for increased affinity. Furthermore, the previously unknown hGLUT1 ligands can serve as chemical tools to further characterize hGLUT1 function or lead molecules for future drug development.
Figures

Similar articles
-
Mechanism of inhibition of human glucose transporter GLUT1 is conserved between cytochalasin B and phenylalanine amides.Proc Natl Acad Sci U S A. 2016 Apr 26;113(17):4711-6. doi: 10.1073/pnas.1603735113. Epub 2016 Apr 12. Proc Natl Acad Sci U S A. 2016. PMID: 27078104 Free PMC article.
-
Mechanistic Insights into Protein Stability and Self-aggregation in GLUT1 Genetic Variants Causing GLUT1-Deficiency Syndrome.J Membr Biol. 2020 Apr;253(2):87-99. doi: 10.1007/s00232-020-00108-3. Epub 2020 Feb 5. J Membr Biol. 2020. PMID: 32025761 Free PMC article. Review.
-
Molecular Dynamics Simulations of the Human Glucose Transporter GLUT1.PLoS One. 2015 Apr 28;10(4):e0125361. doi: 10.1371/journal.pone.0125361. eCollection 2015. PLoS One. 2015. PMID: 25919356 Free PMC article.
-
Conformational Studies of Glucose Transporter 1 (GLUT1) as an Anticancer Drug Target.Molecules. 2019 Jun 7;24(11):2159. doi: 10.3390/molecules24112159. Molecules. 2019. PMID: 31181707 Free PMC article.
-
Pathogenic mutations causing glucose transport defects in GLUT1 transporter: The role of intermolecular forces in protein structure-function.Biophys Chem. 2015 May-Jun;200-201:9-17. doi: 10.1016/j.bpc.2015.03.005. Epub 2015 Mar 25. Biophys Chem. 2015. PMID: 25863194 Review.
Cited by
-
Discovery of New Glucose Uptake Inhibitors as Potential Anticancer Agents by Non-Radioactive Cell-Based Assays.Molecules. 2022 Nov 21;27(22):8106. doi: 10.3390/molecules27228106. Molecules. 2022. PMID: 36432207 Free PMC article.
-
Targeting Glucose Metabolism Enzymes in Cancer Treatment: Current and Emerging Strategies.Cancers (Basel). 2022 Sep 21;14(19):4568. doi: 10.3390/cancers14194568. Cancers (Basel). 2022. PMID: 36230492 Free PMC article. Review.
-
Development of Glucose Transporter (GLUT) Inhibitors.European J Org Chem. 2020 May 3;2020(16):2321-2329. doi: 10.1002/ejoc.201901353. Epub 2019 Nov 28. European J Org Chem. 2020. PMID: 32421048 Free PMC article. Review.
-
Functional and structural analysis of rare SLC2A2 variants associated with Fanconi-Bickel syndrome and metabolic traits.Hum Mutat. 2019 Jul;40(7):983-995. doi: 10.1002/humu.23758. Epub 2019 Apr 25. Hum Mutat. 2019. PMID: 30950137 Free PMC article.
-
Tyrosine Kinase Inhibitors Reduce Glucose Uptake by Binding to an Exofacial Site on hGLUT-1: Influence on 18 F-FDG PET Uptake.Clin Transl Sci. 2021 May;14(3):847-858. doi: 10.1111/cts.12943. Epub 2021 May 3. Clin Transl Sci. 2021. PMID: 33278334 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Chemical Information
Miscellaneous

