Three-year Follow up of GMCSF/bi-shRNA(furin) DNA-transfected Autologous Tumor Immunotherapy (Vigil) in Metastatic Advanced Ewing's Sarcoma
- PMID: 27109631
- PMCID: PMC5023386
- DOI: 10.1038/mt.2016.86
Three-year Follow up of GMCSF/bi-shRNA(furin) DNA-transfected Autologous Tumor Immunotherapy (Vigil) in Metastatic Advanced Ewing's Sarcoma
Abstract
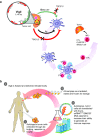
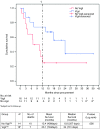
Ewing's sarcoma is a devastating rare pediatric cancer of the bone. Intense chemotherapy temporarily controls disease in most patients at presentation but has limited effect in patients with progressive or recurrent disease. We previously described preliminary results of a novel immunotherapy, FANG (Vigil) vaccine, in which 12 advanced stage Ewing's patients were safely treated and went on to achieve a predicted immune response (IFNγ ELISPOT). We describe follow-up through year 3 of a prospective, nonrandomized study comparing an expanded group of Vigil-treated advanced disease Ewing's sarcoma patients (n = 16) with a contemporaneous group of Ewing's sarcoma patients (n = 14) not treated with Vigil. Long-term follow-up results show a survival benefit without evidence of significant toxicity (no ≥ grade 3) to Vigil when administered once monthly by intradermal injection (1 × 10e(6) cells/injection to 1 × 10e(7) cells/injection). Specifically, we report a 1-year actual survival of 73% for Vigil-treated patients compared to 23% in those not treated with Vigil. In addition, there was a 17.2-month difference in overall survival (OS; Kaplan-Meier) between the Vigil (median OS 731 days) and no Vigil patient groups (median OS 207 days). In conclusion, these results supply the rational for further testing of Vigil in advanced stage Ewing's sarcoma.
Figures
Similar articles
-
Gemogenovatucel-T (Vigil): bi-shRNA plasmid-based targeted immunotherapy.Future Oncol. 2024;20(29):2149-2164. doi: 10.1080/14796694.2024.2376518. Epub 2024 Aug 5. Future Oncol. 2024. PMID: 39101448 Free PMC article. Review.
-
Pilot Study of Recurrent Ewing's Sarcoma Management with Vigil/Temozolomide/Irinotecan and Assessment of Circulating Tumor (ct) DNA.Clin Cancer Res. 2023 May 1;29(9):1689-1697. doi: 10.1158/1078-0432.CCR-22-2292. Clin Cancer Res. 2023. PMID: 36780200 Free PMC article.
-
Phase II study of Vigil® DNA engineered immunotherapy as maintenance in advanced stage ovarian cancer.Gynecol Oncol. 2016 Dec;143(3):504-510. doi: 10.1016/j.ygyno.2016.09.018. Epub 2016 Sep 24. Gynecol Oncol. 2016. PMID: 27678295 Clinical Trial.
-
Pilot Trial of FANG Immunotherapy in Ewing's Sarcoma.Mol Ther. 2015 Jun;23(6):1103-1109. doi: 10.1038/mt.2015.43. Epub 2015 Mar 19. Mol Ther. 2015. PMID: 25917459 Free PMC article.
-
Ewing's sarcoma of the cervix, a diagnostic dilemma: a case report and review of the literature.J Med Case Rep. 2015 Nov 9;9:255. doi: 10.1186/s13256-015-0733-2. J Med Case Rep. 2015. PMID: 26549660 Free PMC article. Review.
Cited by
-
Gemogenovatucel-T (Vigil): bi-shRNA plasmid-based targeted immunotherapy.Future Oncol. 2024;20(29):2149-2164. doi: 10.1080/14796694.2024.2376518. Epub 2024 Aug 5. Future Oncol. 2024. PMID: 39101448 Free PMC article. Review.
-
Emerging mechanisms of immunotherapy resistance in sarcomas.Cancer Drug Resist. 2022 Mar 5;5(1):199-213. doi: 10.20517/cdr.2021.111. eCollection 2022. Cancer Drug Resist. 2022. PMID: 35582530 Free PMC article. Review.
-
Shifting from a Biological-Agnostic Approach to a Molecular-Driven Strategy in Rare Cancers: Ewing Sarcoma Archetype.Biomedicines. 2023 Mar 13;11(3):874. doi: 10.3390/biomedicines11030874. Biomedicines. 2023. PMID: 36979853 Free PMC article. Review.
-
TGF-beta signal transduction: biology, function and therapy for diseases.Mol Biomed. 2022 Dec 19;3(1):45. doi: 10.1186/s43556-022-00109-9. Mol Biomed. 2022. PMID: 36534225 Free PMC article. Review.
-
The proprotein convertase furin in cancer: more than an oncogene.Oncogene. 2022 Feb;41(9):1252-1262. doi: 10.1038/s41388-021-02175-9. Epub 2022 Jan 7. Oncogene. 2022. PMID: 34997216 Review.
References
-
- Esiashvili, N, Goodman, M and Marcus, RB Jr (2008). Changes in incidence and survival of Ewing sarcoma patients over the past 3 decades: Surveillance Epidemiology and End Results data. J Pediatr Hematol Oncol 30: 425–430. - PubMed
-
- http://www.cancer.gov/types/bone/hp/ewing-treatment-pdq. Revised 19 November 2015.
-
- Cotterill, SJ, Ahrens, S, Paulussen, M, Jürgens, HF, Voûte, PA, Gadner, H et al. (2000). Prognostic factors in Ewing's tumor of bone: analysis of 975 patients from the European Intergroup Cooperative Ewing's Sarcoma Study Group. J Clin Oncol 18: 3108–3114. - PubMed
-
- Stahl, M, Ranft, A, Paulussen, M, Bölling, T, Vieth, V, Bielack, S et al. (2011). Risk of recurrence and survival after relapse in patients with Ewing sarcoma. Pediatr Blood Cancer 57: 549–553. - PubMed
-
- Ozaki, T, Hillmann, A, Hoffmann, C, Rübe, C, Blasius, S, Dunst, J et al. (1996). Significance of surgical margin on the prognosis of patients with Ewing's sarcoma. A report from the Cooperative Ewing's Sarcoma Study. Cancer 78: 892–900. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

