An ultrasensitive NanoLuc-based luminescence system for monitoring Plasmodium berghei throughout its life cycle
- PMID: 27102897
- PMCID: PMC4840902
- DOI: 10.1186/s12936-016-1291-9
An ultrasensitive NanoLuc-based luminescence system for monitoring Plasmodium berghei throughout its life cycle
Abstract
Background: Bioluminescence imaging is widely used for cell-based assays and animal imaging studies, both in biomedical research and drug development. Its main advantages include its high-throughput applicability, affordability, high sensitivity, operational simplicity, and quantitative outputs. In malaria research, bioluminescence has been used for drug discovery in vivo and in vitro, exploring host-pathogen interactions, and studying multiple aspects of Plasmodium biology. While the number of fluorescent proteins available for imaging has undergone a great expansion over the last two decades, enabling simultaneous visualization of multiple molecular and cellular events, expansion of available luciferases has lagged behind. The most widely used bioluminescent probe in malaria research is the Photinus pyralis firefly luciferase, followed by the more recently introduced Click-beetle and Renilla luciferases. Ultra-sensitive imaging of Plasmodium at low parasite densities has not been previously achieved. With the purpose of overcoming these challenges, a Plasmodium berghei line expressing the novel ultra-bright luciferase enzyme NanoLuc, called PbNLuc has been generated, and is presented in this work.
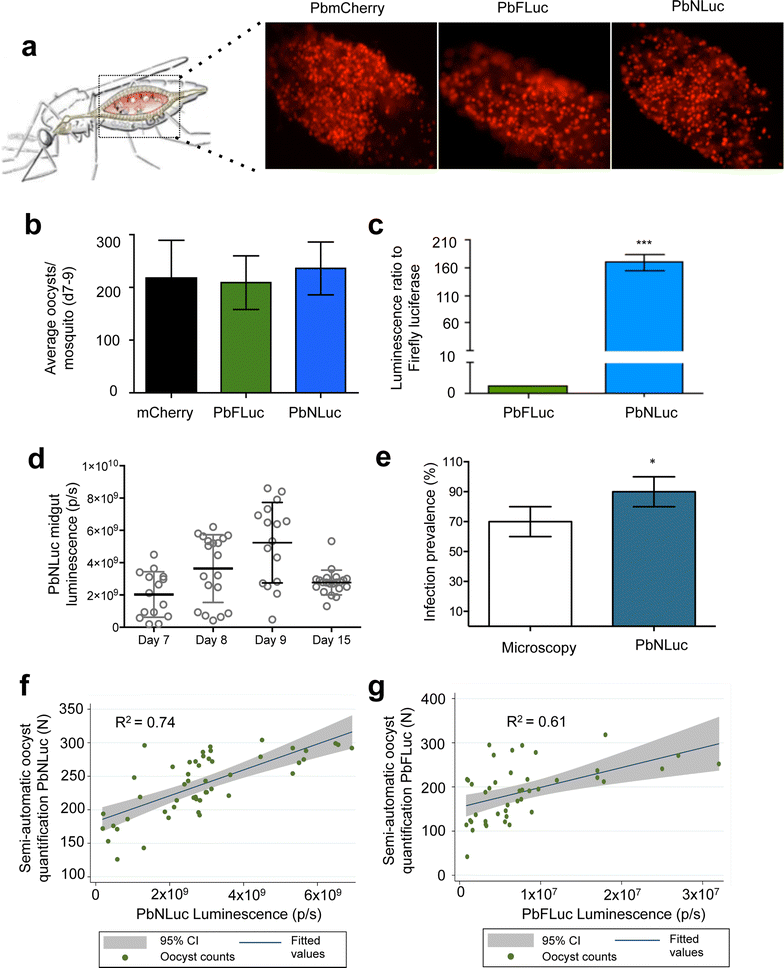
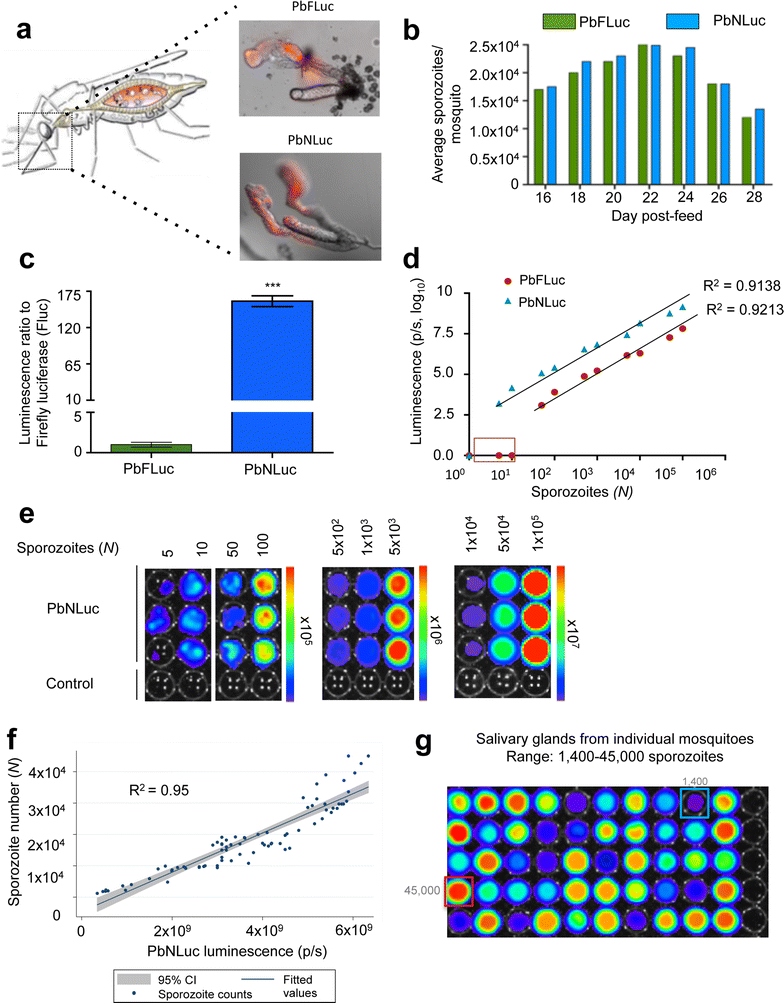
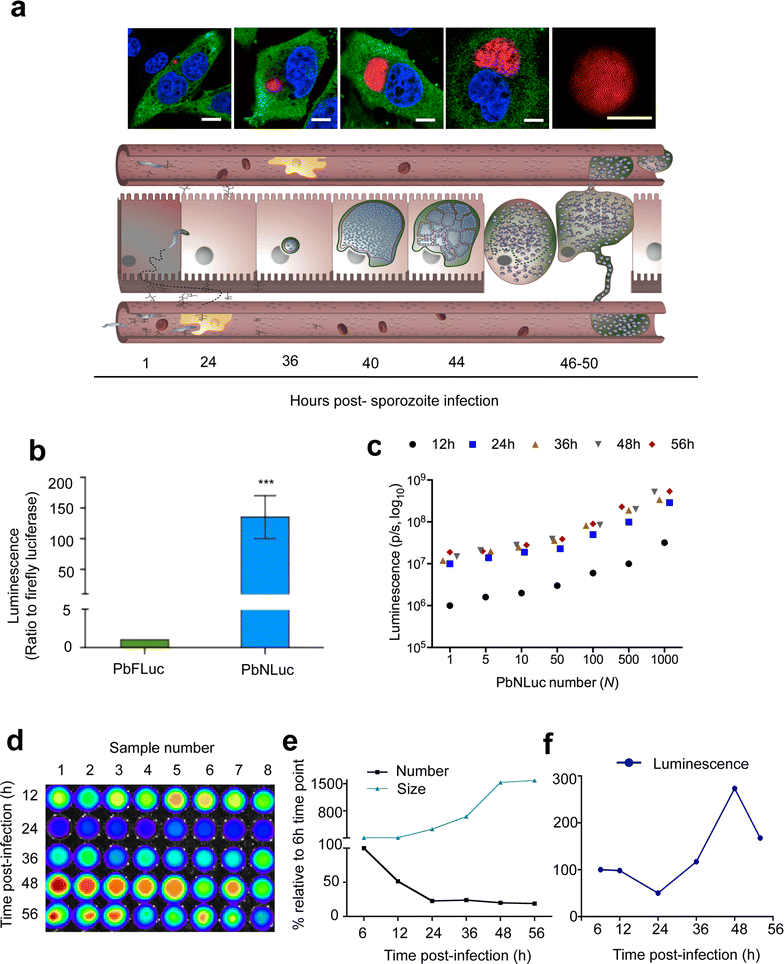
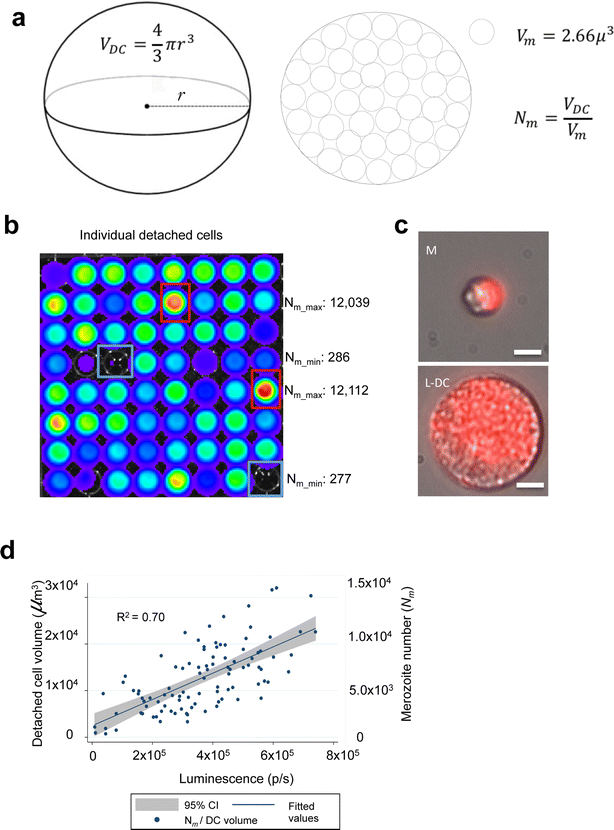
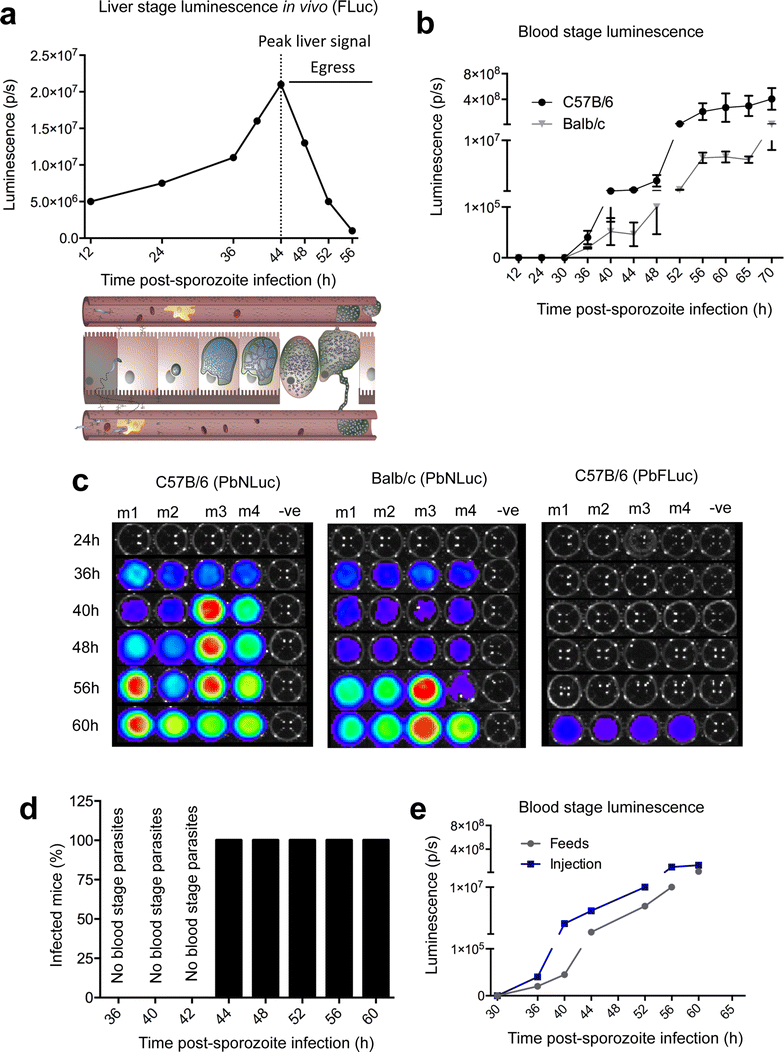
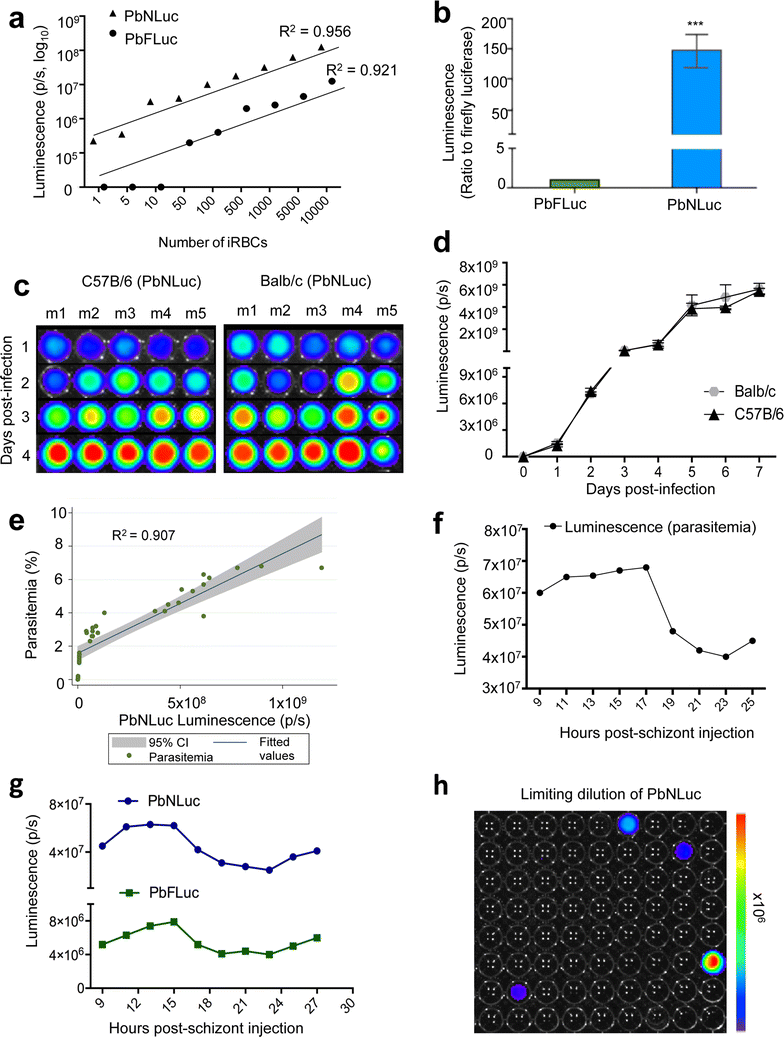
Results: NanoLuc shows at least 150 times brighter signal than firefly luciferase in vitro, allowing single parasite detection in mosquito, liver, and sexual and asexual blood stages. As a proof-of-concept, the PbNLuc parasites were used to image parasite development in the mosquito, liver and blood stages of infection, and to specifically explore parasite liver stage egress, and pre-patency period in vivo.
Conclusions: PbNLuc is a suitable parasite line for sensitive imaging of the entire Plasmodium life cycle. Its sensitivity makes it a promising line to be used as a reference for drug candidate testing, as well as the characterization of mutant parasites to explore the function of parasite proteins, host-parasite interactions, and the better understanding of Plasmodium biology. Since the substrate requirements of NanoLuc are different from those of firefly luciferase, dual bioluminescence imaging for the simultaneous characterization of two lines, or two separate biological processes, is possible, as demonstrated in this work.
Keywords: Bioluminescence; NanoLuc luciferase; PbNLuc; Plasmodium berghei; Ultrabright; Ultrasensitive.
Figures
Similar articles
-
Quantitative analysis of Plasmodium berghei liver stages by bioluminescence imaging.Methods Mol Biol. 2013;923:429-43. doi: 10.1007/978-1-62703-026-7_30. Methods Mol Biol. 2013. PMID: 22990796
-
Real-time in vivo imaging of transgenic bioluminescent blood stages of rodent malaria parasites in mice.Nat Protoc. 2006;1(1):476-85. doi: 10.1038/nprot.2006.69. Nat Protoc. 2006. PMID: 17406270
-
Screening inhibitors of P. berghei blood stages using bioluminescent reporter parasites.Methods Mol Biol. 2013;923:507-22. doi: 10.1007/978-1-62703-026-7_35. Methods Mol Biol. 2013. PMID: 22990801
-
Towards genome-wide experimental genetics in the in vivo malaria model parasite Plasmodium berghei.Pathog Glob Health. 2015 Mar;109(2):46-60. doi: 10.1179/2047773215Y.0000000006. Epub 2015 Mar 19. Pathog Glob Health. 2015. PMID: 25789828 Free PMC article. Review.
-
The private life of malaria parasites: Strategies for sexual reproduction.Mol Biochem Parasitol. 2021 Jul;244:111375. doi: 10.1016/j.molbiopara.2021.111375. Epub 2021 May 20. Mol Biochem Parasitol. 2021. PMID: 34023299 Free PMC article. Review.
Cited by
-
A replication-competent foot-and-mouth disease virus expressing a luciferase reporter.J Virol Methods. 2017 Sep;247:38-44. doi: 10.1016/j.jviromet.2017.05.011. Epub 2017 May 19. J Virol Methods. 2017. PMID: 28532601 Free PMC article.
-
Updating the modified Thompson test by using whole-body bioluminescence imaging to replace traditional efficacy testing in experimental models of murine malaria.Malar J. 2019 Feb 15;18(1):38. doi: 10.1186/s12936-019-2661-x. Malar J. 2019. PMID: 30767768 Free PMC article.
-
Generation of transgenic rodent malaria parasites by transfection of cell culture-derived merozoites.Malar J. 2017 Aug 1;16(1):305. doi: 10.1186/s12936-017-1949-y. Malar J. 2017. PMID: 28764716 Free PMC article.
-
Let it glow: genetically encoded fluorescent reporters in Plasmodium.Malar J. 2024 Apr 20;23(1):114. doi: 10.1186/s12936-024-04936-9. Malar J. 2024. PMID: 38643106 Free PMC article. Review.
-
Torin 2 Derivative, NCATS-SM3710, Has Potent Multistage Antimalarial Activity through Inhibition of P. falciparum Phosphatidylinositol 4-Kinase (Pf PI4KIIIβ).ACS Pharmacol Transl Sci. 2020 Sep 11;3(5):948-964. doi: 10.1021/acsptsci.0c00078. eCollection 2020 Oct 9. ACS Pharmacol Transl Sci. 2020. PMID: 33073193 Free PMC article.
References
-
- WHO . World Malaria Report. Geneva: World Health Organization; 2015.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

