Mechanical Strain Causes Adaptive Change in Bronchial Fibroblasts Enhancing Profibrotic and Inflammatory Responses
- PMID: 27101406
- PMCID: PMC4839664
- DOI: 10.1371/journal.pone.0153926
Mechanical Strain Causes Adaptive Change in Bronchial Fibroblasts Enhancing Profibrotic and Inflammatory Responses
Abstract
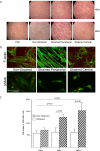
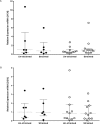
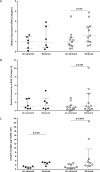
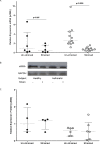
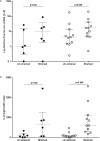
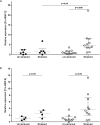
Asthma is characterized by periodic episodes of bronchoconstriction and reversible airway obstruction; these symptoms are attributable to a number of factors including increased mass and reactivity of bronchial smooth muscle and extracellular matrix (ECM) in asthmatic airways. Literature has suggested changes in cell responses and signaling can be elicited via modulation of mechanical stress acting upon them, potentially affecting the microenvironment of the cell. In this study, we hypothesized that mechanical strain directly affects the (myo)fibroblast phenotype in asthma. Therefore, we characterized responses of bronchial fibroblasts, from 6 normal and 11 asthmatic non-smoking volunteers, exposed to cyclical mechanical strain using flexible silastic membranes. Samples were analyzed for proteoglycans, α-smooth muscle actin (αSMA), collagens I and III, matrix metalloproteinase (MMP) 2 & 9 and interleukin-8 (IL-8) by qRT-PCR, Western blot, zymography and ELISA. Mechanical strain caused a decrease in αSMA mRNA but no change in either αSMA protein or proteoglycan expression. In contrast the inflammatory mediator IL-8, MMPs and interstitial collagens were increased at both the transcriptional and protein level. The results demonstrate an adaptive response of bronchial fibroblasts to mechanical strain, irrespective of donor. The adaptation involves cytoskeletal rearrangement, matrix remodelling and inflammatory cytokine release. These results suggest that mechanical strain could contribute to disease progression in asthma by promoting inflammation and remodelling responses.
Conflict of interest statement
Figures
Similar articles
-
Mechanical strain increases cytokine and chemokine production in bronchial fibroblasts from asthmatic patients.Allergy. 2009 Jan;64(1):32-9. doi: 10.1111/j.1398-9995.2008.01814.x. Epub 2008 Dec 12. Allergy. 2009. PMID: 19076933
-
Co-culture of human bronchial fibroblasts and CD4+ T cells increases Th17 cytokine signature.PLoS One. 2013 Dec 5;8(12):e81983. doi: 10.1371/journal.pone.0081983. eCollection 2013. PLoS One. 2013. PMID: 24349168 Free PMC article.
-
Mechanical strain enhances proteoglycan message in fibroblasts from asthmatic subjects.Clin Exp Allergy. 2004 Jun;34(6):926-30. doi: 10.1111/j.1365-2222.2004.01980.x. Clin Exp Allergy. 2004. PMID: 15196281
-
Diagnostic tools assessing airway remodelling in asthma.Allergol Immunopathol (Madr). 2012 Mar-Apr;40(2):108-16. doi: 10.1016/j.aller.2011.11.002. Epub 2012 Jan 9. Allergol Immunopathol (Madr). 2012. PMID: 22236733 Review.
-
Potential role for phenotypic modulation of bronchial smooth muscle cells in chronic asthma.Can J Physiol Pharmacol. 1994 Nov;72(11):1448-57. doi: 10.1139/y94-209. Can J Physiol Pharmacol. 1994. PMID: 7767892 Review.
Cited by
-
Mechanoresponsive regulation of fibroblast-to-myofibroblast transition in three-dimensional tissue analogues: mechanical strain amplitude dependency of fibrosis.Sci Rep. 2022 Oct 7;12(1):16832. doi: 10.1038/s41598-022-20383-5. Sci Rep. 2022. PMID: 36207437 Free PMC article.
-
A Simple Method to Test Mechanical Strain on Epithelial Cell Monolayers Using a 3D-Printed Stretcher.Methods Mol Biol. 2021;2367:235-247. doi: 10.1007/7651_2020_314. Methods Mol Biol. 2021. PMID: 32789778 Free PMC article.
-
Fibroblast-to-myofibroblast transition in bronchial asthma.Cell Mol Life Sci. 2018 Nov;75(21):3943-3961. doi: 10.1007/s00018-018-2899-4. Epub 2018 Aug 12. Cell Mol Life Sci. 2018. PMID: 30101406 Free PMC article. Review.
-
Mechanics of lung cancer: A finite element model shows strain amplification during early tumorigenesis.PLoS Comput Biol. 2022 Oct 24;18(10):e1010153. doi: 10.1371/journal.pcbi.1010153. eCollection 2022 Oct. PLoS Comput Biol. 2022. PMID: 36279309 Free PMC article.
-
Microphysiological systems modeling acute respiratory distress syndrome that capture mechanical force-induced injury-inflammation-repair.APL Bioeng. 2019 Nov 22;3(4):041503. doi: 10.1063/1.5111549. eCollection 2019 Dec. APL Bioeng. 2019. PMID: 31768486 Free PMC article. Review.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

