Wolbachia Blocks Viral Genome Replication Early in Infection without a Transcriptional Response by the Endosymbiont or Host Small RNA Pathways
- PMID: 27089431
- PMCID: PMC4835223
- DOI: 10.1371/journal.ppat.1005536
Wolbachia Blocks Viral Genome Replication Early in Infection without a Transcriptional Response by the Endosymbiont or Host Small RNA Pathways
Erratum in
-
Correction: Wolbachia Blocks Viral Genome Replication Early in Infection without a Transcriptional Response by the Endosymbiont or Host Small RNA Pathways.PLoS Pathog. 2016 May 9;12(5):e1005639. doi: 10.1371/journal.ppat.1005639. eCollection 2016 May. PLoS Pathog. 2016. PMID: 27159401 Free PMC article.
Abstract
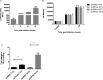
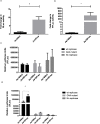
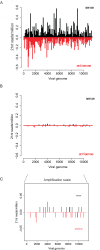
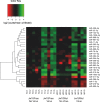
The intracellular endosymbiotic bacterium Wolbachia can protect insects against viral infection, and is being introduced into mosquito populations in the wild to block the transmission of arboviruses that infect humans and are a major public health concern. To investigate the mechanisms underlying this antiviral protection, we have developed a new model system combining Wolbachia-infected Drosophila melanogaster cell culture with the model mosquito-borne Semliki Forest virus (SFV; Togaviridae, Alphavirus). Wolbachia provides strong antiviral protection rapidly after infection, suggesting that an early stage post-infection is being blocked. Wolbachia does appear to have major effects on events distinct from entry, assembly or exit as it inhibits the replication of an SFV replicon transfected into the cells. Furthermore, it causes a far greater reduction in the expression of proteins from the 3' open reading frame than the 5' non-structural protein open reading frame, indicating that it is blocking the replication of viral RNA. Further to this separation of the replicase proteins and viral RNA in transreplication assays shows that uncoupling of viral RNA and replicase proteins does not overcome Wolbachia's antiviral activity. This further suggests that replicative processes are disrupted, such as translation or replication, by Wolbachia infection. This may occur by Wolbachia mounting an active antiviral response, but the virus did not cause any transcriptional response by the bacterium, suggesting that this is not the case. Host microRNAs (miRNAs) have been implicated in protection, but again we found that host cell miRNA expression was unaffected by the bacterium and neither do our findings suggest any involvement of the antiviral siRNA pathway. We conclude that Wolbachia may directly interfere with early events in virus replication such as translation of incoming viral RNA or RNA transcription, and this likely involves an intrinsic (as opposed to an induced) mechanism.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


Similar articles
-
Viral RNA is a target for Wolbachia-mediated pathogen blocking.PLoS Pathog. 2020 Jun 18;16(6):e1008513. doi: 10.1371/journal.ppat.1008513. eCollection 2020 Jun. PLoS Pathog. 2020. PMID: 32555677 Free PMC article.
-
Wolbachia-Conferred Antiviral Protection Is Determined by Developmental Temperature.mBio. 2021 Oct 26;12(5):e0292320. doi: 10.1128/mBio.02923-20. Epub 2021 Sep 7. mBio. 2021. PMID: 34488458 Free PMC article.
-
Wolbachia elevates host methyltransferase expression to block an RNA virus early during infection.PLoS Pathog. 2017 Jun 15;13(6):e1006427. doi: 10.1371/journal.ppat.1006427. eCollection 2017 Jun. PLoS Pathog. 2017. PMID: 28617844 Free PMC article.
-
The Antiviral Effects of the Symbiont Bacteria Wolbachia in Insects.Front Immunol. 2021 Jan 29;11:626329. doi: 10.3389/fimmu.2020.626329. eCollection 2020. Front Immunol. 2021. PMID: 33584729 Free PMC article. Review.
-
Conflict in the Intracellular Lives of Endosymbionts and Viruses: A Mechanistic Look at Wolbachia-Mediated Pathogen-blocking.Viruses. 2018 Mar 21;10(4):141. doi: 10.3390/v10040141. Viruses. 2018. PMID: 29561780 Free PMC article. Review.
Cited by
-
Artificial Selection Finds New Hypotheses for the Mechanism of Wolbachia-Mediated Dengue Blocking in Mosquitoes.Front Microbiol. 2020 Jul 7;11:1456. doi: 10.3389/fmicb.2020.01456. eCollection 2020. Front Microbiol. 2020. PMID: 32733407 Free PMC article.
-
A Review: Wolbachia-Based Population Replacement for Mosquito Control Shares Common Points with Genetically Modified Control Approaches.Pathogens. 2020 May 22;9(5):404. doi: 10.3390/pathogens9050404. Pathogens. 2020. PMID: 32456036 Free PMC article. Review.
-
Variable Inhibition of Zika Virus Replication by Different Wolbachia Strains in Mosquito Cell Cultures.J Virol. 2017 Jun 26;91(14):e00339-17. doi: 10.1128/JVI.00339-17. Print 2017 Jul 15. J Virol. 2017. PMID: 28446677 Free PMC article.
-
Suppression of the pelo protein by Wolbachia and its effect on dengue virus in Aedes aegypti.PLoS Negl Trop Dis. 2018 Apr 11;12(4):e0006405. doi: 10.1371/journal.pntd.0006405. eCollection 2018 Apr. PLoS Negl Trop Dis. 2018. PMID: 29641562 Free PMC article.
-
Transcriptional Response of Wolbachia to Dengue Virus Infection in Cells of the Mosquito Aedes aegypti.mSphere. 2021 Jun 30;6(3):e0043321. doi: 10.1128/mSphere.00433-21. Online ahead of print. mSphere. 2021. PMID: 34190587 Free PMC article.
References
-
- Rainey SM, Shah PN, Kohl A, Dietrich I. Understanding the Wolbachia-mediated inhibition of arboviruses in mosquitoes: progress and challenges. J Gen Virol. 2013;16(10):057422–0. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

