Altered Mucosal Microbiome Diversity and Disease Severity in Sjögren Syndrome
- PMID: 27087247
- PMCID: PMC4834578
- DOI: 10.1038/srep23561
Altered Mucosal Microbiome Diversity and Disease Severity in Sjögren Syndrome
Abstract
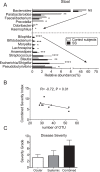
There is mounting evidence that the microbiome has potent immunoregulatory functions. We assessed the effects of intestinal dysbiosis in a model of Sjögren syndrome (SS) by subjecting mice to desiccating stress (DS) and antibiotics (ABX). We characterized the conjunctival, tongue and fecal microbiome profiles of patients with SS. Severity of ocular surface and systemic disease was graded. 16S ribosomal RNA gene sequencing characterized the microbiota. ABX + DS mice had a significantly worse dry eye phenotype compared to controls, a decrease in Clostridium and an increase in Enterobacter, Escherichia/Shigella, and Pseudomonas in stool after ABX + DS for 10 days. Goblet cell density was significantly lower in ABX treated groups compared to controls. Stool from SS subjects had greater relative abundances of Pseudobutyrivibrio, Escherichia/Shigella, Blautia, and Streptococcus, while relative abundance of Bacteroides, Parabacteroides, Faecalibacterium, and Prevotella was reduced compared to controls. The severity of SS ocular and systemic disease was inversely correlated with microbial diversity. These findings suggest that SS is marked by a dysbiotic intestinal microbiome driven by low relative abundance of commensal bacteria and high relative abundance of potentially pathogenic genera that is associated with worse ocular mucosal disease in a mouse model of SS and in SS patients.
Figures

Similar articles
-
Dysbiotic salivary microbiota in dry mouth and primary Sjögren's syndrome patients.PLoS One. 2019 Jun 18;14(6):e0218319. doi: 10.1371/journal.pone.0218319. eCollection 2019. PLoS One. 2019. PMID: 31211815 Free PMC article.
-
Dysbiosis of the buccal mucosa microbiome in primary Sjögren's syndrome patients.Rheumatology (Oxford). 2018 Dec 1;57(12):2225-2234. doi: 10.1093/rheumatology/key215. Rheumatology (Oxford). 2018. PMID: 30060225
-
Connection between the Gut Microbiome, Systemic Inflammation, Gut Permeability and FOXP3 Expression in Patients with Primary Sjögren's Syndrome.Int J Mol Sci. 2020 Nov 19;21(22):8733. doi: 10.3390/ijms21228733. Int J Mol Sci. 2020. PMID: 33228011 Free PMC article.
-
Can Gut Microbiota Affect Dry Eye Syndrome?Int J Mol Sci. 2020 Nov 10;21(22):8443. doi: 10.3390/ijms21228443. Int J Mol Sci. 2020. PMID: 33182758 Free PMC article. Review.
-
A Glimpse Into the Microbiome of Sjögren's Syndrome.Front Immunol. 2022 Jul 14;13:918619. doi: 10.3389/fimmu.2022.918619. eCollection 2022. Front Immunol. 2022. PMID: 35911741 Free PMC article. Review.
Cited by
-
Dry Eye Disease: Emerging Approaches to Disease Analysis and Therapy.J Clin Med. 2019 Sep 11;8(9):1439. doi: 10.3390/jcm8091439. J Clin Med. 2019. PMID: 31514344 Free PMC article. Review.
-
Gut dysbiosis in rheumatic diseases: A systematic review and meta-analysis of 92 observational studies.EBioMedicine. 2022 Jun;80:104055. doi: 10.1016/j.ebiom.2022.104055. Epub 2022 May 17. EBioMedicine. 2022. PMID: 35594658 Free PMC article.
-
The Ocular Microbiome Is Altered by Sampling Modality and Age.Transl Vis Sci Technol. 2021 Oct 4;10(12):24. doi: 10.1167/tvst.10.12.24. Transl Vis Sci Technol. 2021. PMID: 34661621 Free PMC article.
-
Alterations in the gut bacterial microbiome in fungal Keratitis patients.PLoS One. 2018 Jun 22;13(6):e0199640. doi: 10.1371/journal.pone.0199640. eCollection 2018. PLoS One. 2018. PMID: 29933394 Free PMC article.
-
Targeting the Gut-Eye Axis: An Emerging Strategy to Face Ocular Diseases.Int J Mol Sci. 2023 Aug 28;24(17):13338. doi: 10.3390/ijms241713338. Int J Mol Sci. 2023. PMID: 37686143 Free PMC article. Review.
References
-
- Haugen A. J. et al.. Estimation of the prevalence of primary Sjogren’s syndrome in two age-different community-based populations using two sets of classification criteria: the Hordaland Health Study. Scand. J. Rheumatol. 37, 30–34 (2008). - PubMed
-
- Christodoulou M. I., Kapsogeorgou E. K. & Moutsopoulos H. M. Characteristics of the minor salivary gland infiltrates in Sjogren’s syndrome. J. Autoimmun. 34, 400–407 (2010). - PubMed
-
- Pflugfelder S. C. et al.. Conjunctival cytologic features of primary Sjogren’s syndrome. Ophthalmology 97, 985–991 (1990). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

