The final cut: cell polarity meets cytokinesis at the bud neck in S. cerevisiae
- PMID: 27085703
- PMCID: PMC4951512
- DOI: 10.1007/s00018-016-2220-3
The final cut: cell polarity meets cytokinesis at the bud neck in S. cerevisiae
Abstract
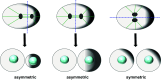
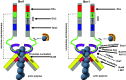
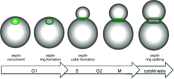
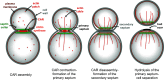
Cell division is a fundamental but complex process that gives rise to two daughter cells. It includes an ordered set of events, altogether called "the cell cycle", that culminate with cytokinesis, the final stage of mitosis leading to the physical separation of the two daughter cells. Symmetric cell division equally partitions cellular components between the two daughter cells, which are therefore identical to one another and often share the same fate. In many cases, however, cell division is asymmetrical and generates two daughter cells that differ in specific protein inheritance, cell size, or developmental potential. The budding yeast Saccharomyces cerevisiae has proven to be an excellent system to investigate the molecular mechanisms governing asymmetric cell division and cytokinesis. Budding yeast is highly polarized during the cell cycle and divides asymmetrically, producing two cells with distinct sizes and fates. Many components of the machinery establishing cell polarization during budding are relocalized to the division site (i.e., the bud neck) for cytokinesis. In this review we recapitulate how budding yeast cells undergo polarized processes at the bud neck for cell division.
Keywords: Actomyosin ring; Budding yeast; Cytokinesis; Formins; Mitotic exit network; Septins.
Figures

Similar articles
-
A safeguard mechanism regulates Rho GTPases to coordinate cytokinesis with the establishment of cell polarity.PLoS Biol. 2013;11(2):e1001495. doi: 10.1371/journal.pbio.1001495. Epub 2013 Feb 26. PLoS Biol. 2013. PMID: 23468594 Free PMC article.
-
Hof1 and Rvs167 have redundant roles in actomyosin ring function during cytokinesis in budding yeast.PLoS One. 2013;8(2):e57846. doi: 10.1371/journal.pone.0057846. Epub 2013 Feb 28. PLoS One. 2013. PMID: 23469085 Free PMC article.
-
Targeting and functional mechanisms of the cytokinesis-related F-BAR protein Hof1 during the cell cycle.Mol Biol Cell. 2013 May;24(9):1305-20. doi: 10.1091/mbc.E12-11-0804. Epub 2013 Mar 6. Mol Biol Cell. 2013. PMID: 23468521 Free PMC article.
-
Septin Organization and Dynamics for Budding Yeast Cytokinesis.J Fungi (Basel). 2024 Sep 9;10(9):642. doi: 10.3390/jof10090642. J Fungi (Basel). 2024. PMID: 39330402 Free PMC article. Review.
-
Mitotic exit and separation of mother and daughter cells.Genetics. 2012 Dec;192(4):1165-202. doi: 10.1534/genetics.112.145516. Genetics. 2012. PMID: 23212898 Free PMC article. Review.
Cited by
-
Genes and lipids that impact uptake and assimilation of exogenous coenzyme Q in Saccharomyces cerevisiae.Free Radic Biol Med. 2020 Jul;154:105-118. doi: 10.1016/j.freeradbiomed.2020.04.029. Epub 2020 May 6. Free Radic Biol Med. 2020. PMID: 32387128 Free PMC article.
-
Candida albicans Cdc15 is essential for mitotic exit and cytokinesis.Sci Rep. 2018 Jun 11;8(1):8899. doi: 10.1038/s41598-018-27157-y. Sci Rep. 2018. PMID: 29891974 Free PMC article.
-
The Mitotic Exit Network Regulates Spindle Pole Body Selection During Sporulation of Saccharomyces cerevisiae.Genetics. 2017 Jun;206(2):919-937. doi: 10.1534/genetics.116.194522. Epub 2017 Apr 26. Genetics. 2017. PMID: 28450458 Free PMC article.
-
The regulation of Net1/Cdc14 by the Hog1 MAPK upon osmostress unravels a new mechanism regulating mitosis.Cell Cycle. 2020 Sep;19(17):2105-2118. doi: 10.1080/15384101.2020.1804222. Epub 2020 Aug 14. Cell Cycle. 2020. PMID: 32794416 Free PMC article. Review.
-
SILAC-based phosphoproteomics reveals new PP2A-Cdc55-regulated processes in budding yeast.Gigascience. 2018 May 1;7(5):giy047. doi: 10.1093/gigascience/giy047. Gigascience. 2018. PMID: 29688323 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

