Targeting the NO/superoxide ratio in adipose tissue: relevance to obesity and diabetes management
- PMID: 27079449
- PMCID: PMC5446578
- DOI: 10.1111/bph.13498
Targeting the NO/superoxide ratio in adipose tissue: relevance to obesity and diabetes management
Abstract
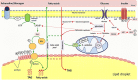
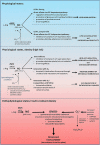
Insulin sensitivity and metabolic homeostasis depend on the capacity of adipose tissue to take up and utilize excess glucose and fatty acids. The key aspects that determine the fuel-buffering capacity of adipose tissue depend on the physiological levels of the small redox molecule, nitric oxide (NO). In addition to impairment of NO synthesis, excessive formation of the superoxide anion (О2•- ) in adipose tissue may be an important interfering factor diverting the signalling of NO and other reactive oxygen and nitrogen species in obesity, resulting in metabolic dysfunction of adipose tissue over time. Besides its role in relief from superoxide burst, enhanced NO signalling may be responsible for the therapeutic benefits of different superoxide dismutase mimetics, in obesity and experimental diabetes models. This review summarizes the role of NO in adipose tissue and highlights the effects of NO/О2•- ratio 'teetering' as a promising pharmacological target in the metabolic syndrome.
Linked articles: This article is part of a themed section on Redox Biology and Oxidative Stress in Health and Disease. To view the other articles in this section visit http://onlinelibrary.wiley.com/doi/10.1111/bph.v174.12/issuetoc.
© 2016 The British Pharmacological Society.
Figures


Similar articles
-
Restoration of perivascular adipose tissue function in diet-induced obese mice without changing bodyweight.Br J Pharmacol. 2017 Oct;174(20):3443-3453. doi: 10.1111/bph.13703. Epub 2017 Jan 31. Br J Pharmacol. 2017. PMID: 28055105 Free PMC article.
-
Increased mitochondrial ROS generation mediates the loss of the anti-contractile effects of perivascular adipose tissue in high-fat diet obese mice.Br J Pharmacol. 2017 Oct;174(20):3527-3541. doi: 10.1111/bph.13687. Epub 2017 Jan 12. Br J Pharmacol. 2017. PMID: 27930804 Free PMC article.
-
The influence of Glucose-dependent Insulinotropic Polypeptide (GIP) on human adipose tissue and fat metabolism: Implications for obesity, type 2 diabetes and Non-Alcoholic Fatty Liver Disease (NAFLD).Peptides. 2020 Mar;125:170208. doi: 10.1016/j.peptides.2019.170208. Epub 2019 Nov 20. Peptides. 2020. PMID: 31759125 Review.
-
Molecular mechanisms regulating perivascular adipose tissue - potential pharmacological targets?Br J Pharmacol. 2017 Oct;174(20):3385-3387. doi: 10.1111/bph.13969. Br J Pharmacol. 2017. PMID: 28940457 Free PMC article.
-
[Insulin resistance: the adipose tissue in the focus].Orv Hetil. 2005 Oct 23;146(43):2199-207. Orv Hetil. 2005. PMID: 16323566 Review. Hungarian.
Cited by
-
ADH5-mediated NO bioactivity maintains metabolic homeostasis in brown adipose tissue.Cell Rep. 2021 Nov 16;37(7):110003. doi: 10.1016/j.celrep.2021.110003. Cell Rep. 2021. PMID: 34788615 Free PMC article.
-
Peroxisomal regulation of redox homeostasis and adipocyte metabolism.Redox Biol. 2019 Jun;24:101167. doi: 10.1016/j.redox.2019.101167. Epub 2019 Mar 14. Redox Biol. 2019. PMID: 30921635 Free PMC article. Review.
-
Targeting vascular (endothelial) dysfunction.Br J Pharmacol. 2017 Jun;174(12):1591-1619. doi: 10.1111/bph.13517. Epub 2016 Jul 4. Br J Pharmacol. 2017. PMID: 27187006 Free PMC article. Review.
-
Nitric Oxide as a Determinant of Human Longevity and Health Span.Int J Mol Sci. 2023 Sep 26;24(19):14533. doi: 10.3390/ijms241914533. Int J Mol Sci. 2023. PMID: 37833980 Free PMC article. Review.
-
Inorganic nitrate, a natural anti-obesity agent: A systematic review and meta-analysis of animal studies.EXCLI J. 2020 Jul 6;19:972-983. doi: 10.17179/excli2020-2515. eCollection 2020. EXCLI J. 2020. PMID: 32788911 Free PMC article. Review.
References
-
- Adida A, Spener F (2006). Adipocyte‐type fatty acid‐binding protein as intercompartmental shuttle for peroxisome proliferator activated receptor gamma agonists in cultured cell. Biochim Biophys Acta 1761: 172–181. - PubMed
-
- Alemany M (2012). Regulation of adipose tissue energy availability through blood flow control in the metabolic syndrome. Free Radic Biol Med 52: 2108–2119. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

