Cellularized Bilayer Pullulan-Gelatin Hydrogel for Skin Regeneration
- PMID: 27072720
- PMCID: PMC4876533
- DOI: 10.1089/ten.TEA.2015.0536
Cellularized Bilayer Pullulan-Gelatin Hydrogel for Skin Regeneration
Abstract
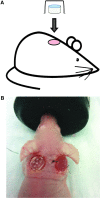
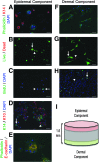
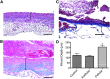
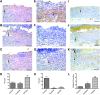
Skin substitutes significantly reduce the morbidity and mortality of patients with burn injuries and chronic wounds. However, current skin substitutes have disadvantages related to high costs and inadequate skin regeneration due to highly inflammatory wounds. Thus, new skin substitutes are needed. By combining two polymers, pullulan, an inexpensive polysaccharide with antioxidant properties, and gelatin, a derivative of collagen with high water absorbency, we created a novel inexpensive hydrogel-named PG-1 for "pullulan-gelatin first generation hydrogel"-suitable for skin substitutes. After incorporating human fibroblasts and keratinocytes onto PG-1 using centrifugation over 5 days, we created a cellularized bilayer skin substitute. Cellularized PG-1 was compared to acellular PG-1 and no hydrogel (control) in vivo in a mouse excisional skin biopsy model using newly developed dome inserts to house the skin substitutes and prevent mouse skin contraction during wound healing. PG-1 had an average pore size of 61.69 μm with an ideal elastic modulus, swelling behavior, and biodegradability for use as a hydrogel for skin substitutes. Excellent skin cell viability, proliferation, differentiation, and morphology were visualized through live/dead assays, 5-bromo-2'-deoxyuridine proliferation assays, and confocal microscopy. Trichrome and immunohistochemical staining of excisional wounds treated with the cellularized skin substitute revealed thicker newly formed skin with a higher proportion of actively proliferating cells and incorporation of human cells compared to acellular PG-1 or control. Excisional wounds treated with acellular or cellularized hydrogels showed significantly less macrophage infiltration and increased angiogenesis 14 days post skin biopsy compared to control. These results show that PG-1 has ideal mechanical characteristics and allows ideal cellular characteristics. In vivo evidence suggests that cellularized PG-1 promotes skin regeneration and may help promote wound healing in highly inflammatory wounds, such as burns and chronic wounds.
Figures


Similar articles
-
Promotion of dermal regeneration using pullulan/gelatin porous skin substitute.J Tissue Eng Regen Med. 2019 Nov;13(11):1965-1977. doi: 10.1002/term.2946. Epub 2019 Aug 8. J Tissue Eng Regen Med. 2019. PMID: 31350941 Free PMC article.
-
(+)4-cholesten-3-one/sodium alginate/gelatin hydrogel for full-thickness wound repair and skin regeneration.Biomed Mater. 2024 Aug 8;19(5). doi: 10.1088/1748-605X/ad6966. Biomed Mater. 2024. PMID: 39114907
-
[Properties of gelatin-polyethylene glycol hydrogel loaded with silver nanoparticle Chlorella and its effects on healing of infected full-thickness skin defect wounds in mice].Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2024 Jan 20;40(1):33-42. doi: 10.3760/cma.j.cn501225-20231020-00126. Zhonghua Shao Shang Yu Chuang Mian Xiu Fu Za Zhi. 2024. PMID: 38296235 Chinese.
-
Advances in gelatin-based hydrogels for wound management.J Mater Chem B. 2021 Feb 14;9(6):1503-1520. doi: 10.1039/d0tb02582h. Epub 2021 Jan 20. J Mater Chem B. 2021. PMID: 33470270 Review.
-
Advanced Hydrogels as Wound Dressings.Biomolecules. 2020 Aug 11;10(8):1169. doi: 10.3390/biom10081169. Biomolecules. 2020. PMID: 32796593 Free PMC article. Review.
Cited by
-
Promotion of dermal regeneration using pullulan/gelatin porous skin substitute.J Tissue Eng Regen Med. 2019 Nov;13(11):1965-1977. doi: 10.1002/term.2946. Epub 2019 Aug 8. J Tissue Eng Regen Med. 2019. PMID: 31350941 Free PMC article.
-
Engineered Pullulan-Collagen-Gold Nano Composite Improves Mesenchymal Stem Cells Neural Differentiation and Inflammatory Regulation.Cells. 2021 Nov 23;10(12):3276. doi: 10.3390/cells10123276. Cells. 2021. PMID: 34943784 Free PMC article.
-
Electrospinning Live Cells Using Gelatin and Pullulan.Bioengineering (Basel). 2020 Feb 22;7(1):21. doi: 10.3390/bioengineering7010021. Bioengineering (Basel). 2020. PMID: 32098366 Free PMC article.
-
Development of a bacterial cellulose-based hydrogel cell carrier containing keratinocytes and fibroblasts for full-thickness wound healing.Sci Rep. 2018 Feb 13;8(1):2875. doi: 10.1038/s41598-018-21174-7. Sci Rep. 2018. PMID: 29440678 Free PMC article.
-
Pullulan-Based Hydrogels in Wound Healing and Skin Tissue Engineering Applications: A Review.Int J Mol Sci. 2023 Mar 4;24(5):4962. doi: 10.3390/ijms24054962. Int J Mol Sci. 2023. PMID: 36902394 Free PMC article. Review.
References
-
- Greaves N.S., Iqbal S.A., Baguneid M., and Bayat A. The role of skin substitutes in the management of chronic cutaneous wounds. Wound Repair Regen 21, 194, 2013 - PubMed
-
- Nyame T.T., Chiang H.A., and Orgill D.P. Clinical applications of skin substitutes. Surg Clin North Am 94, 839, 2014 - PubMed
-
- Bello Y.M., Falabella A.F., and Eaglstein W.H. Tissue-engineered skin. Current status in wound healing. Am J Clin Dermatol 2, 305, 2001 - PubMed
-
- Rawlingson A. Nitric oxide, inflammation and acute burn injury. Burns 29, 631, 2003 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

