Function and regulation of TRPP2 ion channel revealed by a gain-of-function mutant
- PMID: 27071085
- PMCID: PMC4855601
- DOI: 10.1073/pnas.1517066113
Function and regulation of TRPP2 ion channel revealed by a gain-of-function mutant
Abstract
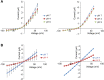
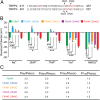
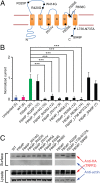
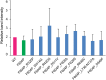
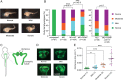
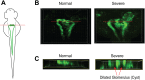
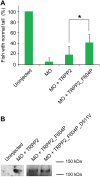
Mutations in polycystin-1 and transient receptor potential polycystin 2 (TRPP2) account for almost all clinically identified cases of autosomal dominant polycystic kidney disease (ADPKD), one of the most common human genetic diseases. TRPP2 functions as a cation channel in its homomeric complex and in the TRPP2/polycystin-1 receptor/ion channel complex. The activation mechanism of TRPP2 is unknown, which significantly limits the study of its function and regulation. Here, we generated a constitutively active gain-of-function (GOF) mutant of TRPP2 by applying a mutagenesis scan on the S4-S5 linker and the S5 transmembrane domain, and studied functional properties of the GOF TRPP2 channel. We found that extracellular divalent ions, including Ca(2+), inhibit the permeation of monovalent ions by directly blocking the TRPP2 channel pore. We also found that D643, a negatively charged amino acid in the pore, is crucial for channel permeability. By introducing single-point ADPKD pathogenic mutations into the GOF TRPP2, we showed that different mutations could have completely different effects on channel activity. The in vivo function of the GOF TRPP2 was investigated in zebrafish embryos. The results indicate that, compared with wild type (WT), GOF TRPP2 more efficiently rescued morphological abnormalities, including curly tail and cyst formation in the pronephric kidney, caused by down-regulation of endogenous TRPP2 expression. Thus, we established a GOF TRPP2 channel that can serve as a powerful tool for studying the function and regulation of TRPP2. The GOF channel may also have potential application for developing new therapeutic strategies for ADPKD.
Keywords: TRP channels; TRPP2; autosomal dominant polycystic kidney disease; gain of function; polycystin.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



Similar articles
-
Extracellular Loops Are Essential for the Assembly and Function of Polycystin Receptor-Ion Channel Complexes.J Biol Chem. 2017 Mar 10;292(10):4210-4221. doi: 10.1074/jbc.M116.767897. Epub 2017 Feb 2. J Biol Chem. 2017. PMID: 28154010 Free PMC article.
-
The ion channel function of polycystin-1 in the polycystin-1/polycystin-2 complex.EMBO Rep. 2019 Nov 5;20(11):e48336. doi: 10.15252/embr.201948336. Epub 2019 Aug 22. EMBO Rep. 2019. PMID: 31441214 Free PMC article.
-
Function and regulation of TRPP2 at the plasma membrane.Am J Physiol Renal Physiol. 2009 Jul;297(1):F1-9. doi: 10.1152/ajprenal.90277.2008. Epub 2009 Feb 25. Am J Physiol Renal Physiol. 2009. PMID: 19244406 Free PMC article. Review.
-
Polycystin-2 (TRPP2): Ion channel properties and regulation.Gene. 2022 Jun 15;827:146313. doi: 10.1016/j.gene.2022.146313. Epub 2022 Mar 18. Gene. 2022. PMID: 35314260 Review.
-
Naringenin inhibits the growth of Dictyostelium and MDCK-derived cysts in a TRPP2 (polycystin-2)-dependent manner.Br J Pharmacol. 2014 May;171(10):2659-70. doi: 10.1111/bph.12443. Br J Pharmacol. 2014. PMID: 24116661 Free PMC article.
Cited by
-
The Polysite Pharmacology of TREK K2P Channels.Adv Exp Med Biol. 2021;1349:51-65. doi: 10.1007/978-981-16-4254-8_4. Adv Exp Med Biol. 2021. PMID: 35138610 Free PMC article.
-
Metformin Inhibits Cyst Formation in a Zebrafish Model of Polycystin-2 Deficiency.Sci Rep. 2017 Aug 2;7(1):7161. doi: 10.1038/s41598-017-07300-x. Sci Rep. 2017. PMID: 28769124 Free PMC article.
-
Establishment of transgenic pigs overexpressing human PKD2-D511V mutant.Front Genet. 2022 Nov 14;13:1059682. doi: 10.3389/fgene.2022.1059682. eCollection 2022. Front Genet. 2022. PMID: 36452154 Free PMC article.
-
Calcium Homeostasis, Transporters, and Blockers in Health and Diseases of the Cardiovascular System.Int J Mol Sci. 2023 May 15;24(10):8803. doi: 10.3390/ijms24108803. Int J Mol Sci. 2023. PMID: 37240147 Free PMC article. Review.
-
Role of PKD2 in the endoplasmic reticulum calcium homeostasis.Front Physiol. 2022 Aug 10;13:962571. doi: 10.3389/fphys.2022.962571. eCollection 2022. Front Physiol. 2022. PMID: 36035467 Free PMC article. Review.
References
-
- Wu G, Somlo S. Molecular genetics and mechanism of autosomal dominant polycystic kidney disease. Mol Genet Metab. 2000;69(1):1–15. - PubMed
-
- Semmo M, Köttgen M, Hofherr A. The TRPP subfamily and polycystin-1 proteins. Handbook Exp Pharmacol. 2014;222:675–711. - PubMed
-
- Hughes J, et al. The polycystic kidney disease 1 (PKD1) gene encodes a novel protein with multiple cell recognition domains. Nat Genet. 1995;10(2):151–160. - PubMed
-
- Mochizuki T, et al. PKD2, a gene for polycystic kidney disease that encodes an integral membrane protein. Science. 1996;272(5266):1339–1342. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

