The Anti-fibrotic Actions of Relaxin Are Mediated Through a NO-sGC-cGMP-Dependent Pathway in Renal Myofibroblasts In Vitro and Enhanced by the NO Donor, Diethylamine NONOate
- PMID: 27065874
- PMCID: PMC4815292
- DOI: 10.3389/fphar.2016.00091
The Anti-fibrotic Actions of Relaxin Are Mediated Through a NO-sGC-cGMP-Dependent Pathway in Renal Myofibroblasts In Vitro and Enhanced by the NO Donor, Diethylamine NONOate
Abstract
Introduction: The anti-fibrotic hormone, relaxin, has been inferred to disrupt transforming growth factor (TGF)-β1/Smad2 phosphorylation (pSmad2) signal transduction and promote collagen-degrading gelatinase activity via a nitric oxide (NO)-dependent pathway. Here, we determined the extent to which NO, soluble guanylate cyclase (sGC) and cyclic guanosine monophosphate (cGMP) were directly involved in the anti-fibrotic actions of relaxin using a selective NO scavenger and sGC inhibitor, and comparing and combining relaxin's effects with that of an NO donor.
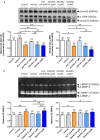
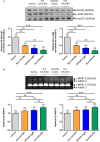
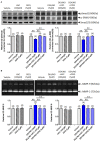
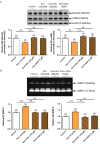
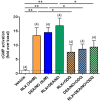
Methods and results: Primary renal cortical myofibroblasts isolated from injured rat kidneys were treated with human recombinant relaxin (RLX; 16.8 nM), the NO donor, diethylamine NONOate (DEA/NO; 0.5-5 μM) or the combined effects of RLX (16.8 nM) and DEA/NO (5 μM) over 72 h. The effects of RLX (16.8 nM) and DEA/NO (5 μM) were also evaluated in the presence of the NO scavenger, hydroxocobalamin (HXC; 100 μM) or sGC inhibitor, ODQ (5 μM) over 72 h. Furthermore, the effects of RLX (30 nM), DEA/NO (5 μM) and RLX (30 nM) + DEA/NO (5 μM) on cGMP levels were directly measured, in the presence or absence of ODQ (5 μM). Changes in matrix metalloproteinase (MMP)-2, MMP-9 (cell media), pSmad2 and α-smooth muscle actin (α-SMA; a measure myofibroblast differentiation) (cell layer) were assessed by gelatin zymography and Western blotting, respectively. At the highest concentration tested, both RLX and DEA/NO promoted MMP-2 and MMP-9 levels by 25-33%, while inhibiting pSmad2 and α-SMA expression by up to 50% (all p < 0.05 vs. untreated and vehicle-treated cells). However, 5μM of DEA/NO was required to produce the effects seen with 16.8 nM of RLX over 72 h. The anti-fibrotic effects of RLX or DEA/NO alone were completely abrogated by HXC and ODQ (both p < 0.01 vs. RLX alone or DEA/NO alone), but were significantly enhanced when added in combination (all p < 0.05 vs. RLX alone). Additionally, the direct cGMP-promoting effects of RLX, DEA/NO and RLX+DEA/NO (which all increased cGMP levels by 12-16-fold over basal levels; all p < 0.01 vs. vehicle-treated cells) were significantly inhibited by pre-treatment of ODQ (all p < 0.05 vs. the respective treatments alone).
Conclusion: These findings confirmed that RLX mediates its TGF-β1-inhibitory and gelatinase-promoting effects via a NO-sGC-cGMP-dependent pathway, which was additively augmented by co-administration of DEA/NO.
Keywords: cGMP; cell signaling; fibrosis; myofibroblasts; nitric oxide; relaxin; transforming growth factor-β1.
Figures
Similar articles
-
Relaxin signals through a RXFP1-pERK-nNOS-NO-cGMP-dependent pathway to up-regulate matrix metalloproteinases: the additional involvement of iNOS.PLoS One. 2012;7(8):e42714. doi: 10.1371/journal.pone.0042714. Epub 2012 Aug 22. PLoS One. 2012. PMID: 22936987 Free PMC article.
-
The anti-fibrotic actions of relaxin are mediated through AT2 R-associated protein phosphatases via RXFP1-AT2 R functional crosstalk in human cardiac myofibroblasts.FASEB J. 2020 Jun;34(6):8217-8233. doi: 10.1096/fj.201902506R. Epub 2020 Apr 16. FASEB J. 2020. PMID: 32297670
-
Relaxin prevents cardiac fibroblast-myofibroblast transition via notch-1-mediated inhibition of TGF-β/Smad3 signaling.PLoS One. 2013 May 21;8(5):e63896. doi: 10.1371/journal.pone.0063896. Print 2013. PLoS One. 2013. PMID: 23704950 Free PMC article.
-
The Potential of sGC Modulators for the Treatment of Age-Related Fibrosis: A Mini-Review.Gerontology. 2017;63(3):216-227. doi: 10.1159/000450946. Epub 2016 Oct 27. Gerontology. 2017. PMID: 27784018 Review.
-
Investigations into the inhibitory effects of relaxin on renal myofibroblast differentiation.Ann N Y Acad Sci. 2009 Apr;1160:294-9. doi: 10.1111/j.1749-6632.2008.03823.x. Ann N Y Acad Sci. 2009. PMID: 19416207 Review.
Cited by
-
ML290 is a biased allosteric agonist at the relaxin receptor RXFP1.Sci Rep. 2017 Jun 7;7(1):2968. doi: 10.1038/s41598-017-02916-5. Sci Rep. 2017. PMID: 28592882 Free PMC article.
-
Study on the mechanism of aging-related erectile dysfunction based on bioinformatics and experimental verification.Transl Androl Urol. 2023 Feb 28;12(2):197-208. doi: 10.21037/tau-22-511. Epub 2023 Feb 3. Transl Androl Urol. 2023. PMID: 36915879 Free PMC article.
-
Relaxin-2 therapy reverses radiation-induced fibrosis and restores bladder function in mice.Neurourol Urodyn. 2018 Nov;37(8):2441-2451. doi: 10.1002/nau.23721. Epub 2018 May 28. Neurourol Urodyn. 2018. PMID: 29806709 Free PMC article.
-
Involvement of Cyclic Guanosine Monophosphate-Dependent Protein Kinase I in Renal Antifibrotic Effects of Serelaxin.Front Pharmacol. 2016 Jul 12;7:195. doi: 10.3389/fphar.2016.00195. eCollection 2016. Front Pharmacol. 2016. PMID: 27462268 Free PMC article.
-
Intraarticular injection of relaxin-2 alleviates shoulder arthrofibrosis.Proc Natl Acad Sci U S A. 2019 Jun 18;116(25):12183-12192. doi: 10.1073/pnas.1900355116. Epub 2019 Jun 3. Proc Natl Acad Sci U S A. 2019. PMID: 31160441 Free PMC article.
References
-
- Aimes R. T., Quigley J. P. (1995). Matrix metalloproteinase-2 is an interstitial collagenase. Inhibitor-free enzyme catalyzes the cleavage of collagen fibrils and soluble native type I collagen generating the specific 3/4- and 1/4-length fragments. J. Biol. Chem. 270 5872–5876. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

