High efficacy of the BCL-2 inhibitor ABT199 (venetoclax) in BCL-2 high-expressing neuroblastoma cell lines and xenografts and rational for combination with MCL-1 inhibition
- PMID: 27056887
- PMCID: PMC5053701
- DOI: 10.18632/oncotarget.8547
High efficacy of the BCL-2 inhibitor ABT199 (venetoclax) in BCL-2 high-expressing neuroblastoma cell lines and xenografts and rational for combination with MCL-1 inhibition
Abstract
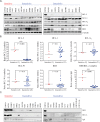
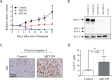
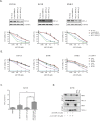
The anti-apoptotic protein B cell lymphoma/leukaemia 2 (BCL-2) is highly expressed in neuroblastoma and plays an important role in oncogenesis. In this study, the selective BCL-2 inhibitor ABT199 was tested in a panel of neuroblastoma cell lines with diverse expression levels of BCL-2 and other BCL-2 family proteins. ABT199 caused apoptosis more potently in neuroblastoma cell lines expressing high BCL-2 and BIM/BCL-2 complex levels than low expressing cell lines. Effects on cell viability correlated with effects on BIM displacement from BCL-2 and cytochrome c release from the mitochondria. ABT199 treatment of mice with neuroblastoma tumors expressing high BCL-2 levels only resulted in growth inhibition, despite maximum BIM displacement from BCL-2 and the induction of a strong apoptotic response. We showed that neuroblastoma cells might survive ABT199 treatment due to its acute upregulation of the anti-apoptotic BCL-2 family protein myeloid cell leukaemia sequence 1 (MCL-1) and BIM sequestration by MCL-1. In vitro inhibition of MCL-1 sensitized neuroblastoma cell lines to ABT199, confirming the pivotal role of MCL-1 in ABT199 resistance. Our findings suggest that neuroblastoma patients with high BCL-2 and BIM/BCL-2 complex levels might benefit from combination treatment with ABT199 and compounds that inhibit MCL-1 expression.
Keywords: ABT199; BCL-2; MCL-1; neuroblastoma; resistance.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


Similar articles
-
Superior anti-tumor activity of the MDM2 antagonist idasanutlin and the Bcl-2 inhibitor venetoclax in p53 wild-type acute myeloid leukemia models.J Hematol Oncol. 2016 Jun 28;9(1):50. doi: 10.1186/s13045-016-0280-3. J Hematol Oncol. 2016. PMID: 27353420 Free PMC article.
-
Select Bcl-2 antagonism restores chemotherapy sensitivity in high-risk neuroblastoma.BMC Cancer. 2016 Feb 13;16:97. doi: 10.1186/s12885-016-2129-0. BMC Cancer. 2016. PMID: 26874859 Free PMC article.
-
MCL-1 and BCL-xL-dependent resistance to the BCL-2 inhibitor ABT-199 can be overcome by preventing PI3K/AKT/mTOR activation in lymphoid malignancies.Cell Death Dis. 2015 Jan 15;6(1):e1593. doi: 10.1038/cddis.2014.525. Cell Death Dis. 2015. PMID: 25590803 Free PMC article.
-
Pathways and mechanisms of venetoclax resistance.Leuk Lymphoma. 2017 Sep;58(9):1-17. doi: 10.1080/10428194.2017.1283032. Epub 2017 Jan 31. Leuk Lymphoma. 2017. PMID: 28140720 Free PMC article. Review.
-
Therapeutics targeting Bcl-2 in hematological malignancies.Biochem J. 2017 Oct 23;474(21):3643-3657. doi: 10.1042/BCJ20170080. Biochem J. 2017. PMID: 29061914 Review.
Cited by
-
Venetoclax-based Rational Combinations are Effective in Models of MYCN-amplified Neuroblastoma.Mol Cancer Ther. 2021 Aug;20(8):1400-1411. doi: 10.1158/1535-7163.MCT-20-0710. Epub 2021 Jun 4. Mol Cancer Ther. 2021. PMID: 34088831 Free PMC article.
-
Recombinant Newcastle disease virus expressing human IFN-λ1 (rL-hIFN-λ1)-induced apoptosis of A549 cells is connected to endoplasmic reticulum stress pathways.Thorac Cancer. 2018 Nov;9(11):1437-1452. doi: 10.1111/1759-7714.12857. Epub 2018 Sep 23. Thorac Cancer. 2018. PMID: 30246439 Free PMC article.
-
The small molecule Bcl-2/Mcl-1 inhibitor TW-37 shows single-agent cytotoxicity in neuroblastoma cell lines.BMC Cancer. 2019 Mar 18;19(1):243. doi: 10.1186/s12885-019-5439-1. BMC Cancer. 2019. PMID: 30885150 Free PMC article.
-
Dual targeting of MDM2 and BCL2 as a therapeutic strategy in neuroblastoma.Oncotarget. 2017 Jul 4;8(34):57047-57057. doi: 10.18632/oncotarget.18982. eCollection 2017 Aug 22. Oncotarget. 2017. PMID: 28915653 Free PMC article.
-
Maternal embryonic leucine zipper kinase is a novel target for diffuse large B cell lymphoma and mantle cell lymphoma.Blood Cancer J. 2019 Nov 18;9(12):87. doi: 10.1038/s41408-019-0249-x. Blood Cancer J. 2019. PMID: 31740676 Free PMC article.
References
-
- Yip KW, Reed JC. Bcl-2 family proteins and cancer. Oncogene. 2008;27:6398–6406. - PubMed
-
- Czabotar PE, Lessene G, Strasser A, Adams JM. Control of apoptosis by the BCL-2 protein family: implications for physiology and therapy. Nat Rev Mol Cell Biol. 2014;15:49–63. - PubMed
-
- Iqbal J, Neppalli VT, Wright G, Dave BJ, Horsman DE, Rosenwald A, Lynch J, Hans CP, Weisenburger DD, Greiner TC, Gascoyne RD, Campo E, Ott G, Muller-Hermelink HK, Delabie J, Jaffe ES, et al. BCL2 expression is a prognostic marker for the activated B-cell-like type of diffuse large B-cell lymphoma. J Clin Oncol. 2006;24:961–968. - PubMed
-
- Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

