Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane
- PMID: 27056669
- PMCID: PMC4840302
- DOI: 10.1101/gad.276725.115
Inactivation of prosurvival Bcl-2 proteins activates Bax/Bak through the outer mitochondrial membrane
Abstract
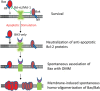
The mechanism of Bax/Bak activation remains a central question in mitochondria-dependent apoptotic signaling. While it is established that all proapoptotic Bcl-2 homology 3 (BH3)-only proteins bind and neutralize the anti-apoptotic Bcl-2 family proteins, how this neutralization leads to Bax/Bak activation has been actively debated. Here, genome editing was used to generate cells deficient for all eight proapoptotic BH3-only proteins (OctaKO) and those that lack the entire Bcl-2 family (Bcl-2 allKO). Although the OctaKO cells were resistant to most apoptotic stimuli tested, they underwent Bax/Bak-dependent and p53/Rb-independent apoptosis efficiently when both Bcl-xL and Mcl-1, two anti-apoptotic Bcl-2 proteins, were inactivated or eliminated. Strikingly, when expressed in the Bcl-2 allKO cells, both Bax and Bak spontaneously associated with the outer mitochondrial membrane (OMM) through their respective helix 9, and this association triggered their homo-oligomerization/activation. Together, these results strongly suggest that the OMM, not BH3-only proteins or p53/Rb, is the long-sought-after direct activator of Bax/Bak following BH3-only-mediated neutralization of anti-apoptotic Bcl-2 proteins.
Keywords: BH3-only; Bax/Bak activation; anti-apoptotic Bcl-2 proteins; outer mitochondrial membrane.
© 2016 O'Neill et al.; Published by Cold Spring Harbor Laboratory Press.
Figures





Similar articles
-
Bax/Bak activation in the absence of Bid, Bim, Puma, and p53.Cell Death Dis. 2016 Jun 16;7(6):e2266. doi: 10.1038/cddis.2016.167. Cell Death Dis. 2016. PMID: 27310874 Free PMC article.
-
BH3-only proteins target BCL-xL/MCL-1, not BAX/BAK, to initiate apoptosis.Cell Res. 2019 Nov;29(11):942-952. doi: 10.1038/s41422-019-0231-y. Epub 2019 Sep 24. Cell Res. 2019. PMID: 31551537 Free PMC article.
-
MOMP in the absence of BH3-only proteins.Genes Dev. 2016 Apr 15;30(8):878-80. doi: 10.1101/gad.281519.116. Genes Dev. 2016. PMID: 27083995 Free PMC article.
-
BCL-2 proteins and apoptosis: Recent insights and unknowns.Biochem Biophys Res Commun. 2018 May 27;500(1):26-34. doi: 10.1016/j.bbrc.2017.06.190. Epub 2017 Jul 1. Biochem Biophys Res Commun. 2018. PMID: 28676391 Review.
-
Pro-apoptotic complexes of BAX and BAK on the outer mitochondrial membrane.Biochim Biophys Acta Mol Cell Res. 2022 Oct;1869(10):119317. doi: 10.1016/j.bbamcr.2022.119317. Epub 2022 Jun 22. Biochim Biophys Acta Mol Cell Res. 2022. PMID: 35752202 Review.
Cited by
-
Physiological and transcriptomic analysis dissects the molecular mechanism governing meat quality during postmortem aging in Hu sheep (Ovis aries).Front Nutr. 2024 Jan 5;10:1321938. doi: 10.3389/fnut.2023.1321938. eCollection 2023. Front Nutr. 2024. PMID: 38249602 Free PMC article.
-
Mitotic slippage: an old tale with a new twist.Cell Cycle. 2019 Jan;18(1):7-15. doi: 10.1080/15384101.2018.1559557. Epub 2019 Jan 2. Cell Cycle. 2019. PMID: 30601084 Free PMC article. Review.
-
CDK5 Inhibitor Downregulates Mcl-1 and Sensitizes Pancreatic Cancer Cell Lines to Navitoclax.Mol Pharmacol. 2019 Oct;96(4):419-429. doi: 10.1124/mol.119.116855. Mol Pharmacol. 2019. PMID: 31467029 Free PMC article.
-
Shedding Light on the Interaction of Human Anti-Apoptotic Bcl-2 Protein with Ligands through Biophysical and in Silico Studies.Int J Mol Sci. 2019 Feb 16;20(4):860. doi: 10.3390/ijms20040860. Int J Mol Sci. 2019. PMID: 30781512 Free PMC article.
-
Lactobacillus casei Strain Shirota Enhances the In Vitro Antiproliferative Effect of Geniposide in Human Oral Squamous Carcinoma HSC-3 Cells.Molecules. 2018 May 3;23(5):1069. doi: 10.3390/molecules23051069. Molecules. 2018. PMID: 29751513 Free PMC article.
References
-
- Brouwer JM, Westphal D, Dewson G, Robin AY, Uren RT, Bartolo R, Thompson GV, Colman PM, Kluck RM, Czabotar PE. 2014. Bak core and latch domains separate during activation, and freed core domains form symmetric homodimers. Mol Cell 55: 938–946. - PubMed
-
- Chen L, Willis SN, Wei A, Smith BJ, Fletcher JI, Hinds MG, Colman PM, Day CL, Adams JM, Huang DC. 2005. Differential targeting of prosurvival Bcl-2 proteins by their BH3-only ligands allows complementary apoptotic function. Mol Cell 17: 393–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous
