Calcium-sensing receptors signal constitutive macropinocytosis and facilitate the uptake of NOD2 ligands in macrophages
- PMID: 27050483
- PMCID: PMC4823870
- DOI: 10.1038/ncomms11284
Calcium-sensing receptors signal constitutive macropinocytosis and facilitate the uptake of NOD2 ligands in macrophages
Abstract
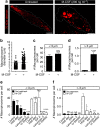
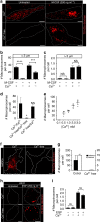
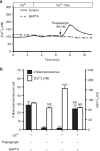
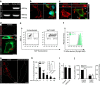
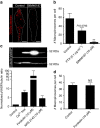
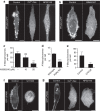
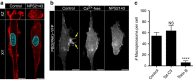
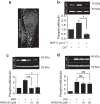
Macropinocytosis can be induced in several cell types by stimulation with growth factors. In selected cell types, notably macrophages and dendritic cells, macropinocytosis occurs constitutively, supporting the uptake of antigens for subsequent presentation. Despite their different mode of initiation and contrasting physiological roles, it is tacitly assumed that both types of macropinocytosis are mechanistically identical. We report that constitutive macropinocytosis is stringently calcium dependent, while stimulus-induced macropinocytosis is not. Extracellular calcium is sensed by G-protein-coupled calcium-sensing receptors (CaSR) that signal macropinocytosis through Gα-, phosphatidylinositol 3-kinase and phospholipase C. These pathways promote the recruitment of exchange factors that stimulate Rac and/or Cdc42, driving actin-dependent formation of ruffles and macropinosomes. In addition, the heterologous expression of CaSR in HEK293 cells confers on them the ability to perform constitutive macropinocytosis. Finally, we show that CaSR-induced constitutive macropinocytosis facilitates the sentinel function of macrophages, promoting the efficient delivery of ligands to cytosolic pattern-recognition receptors.
Figures
Similar articles
-
Nox2-Mediated PI3K and Cofilin Activation Confers Alternate Redox Control of Macrophage Pinocytosis.Antioxid Redox Signal. 2017 Jun 1;26(16):902-916. doi: 10.1089/ars.2016.6639. Epub 2016 Sep 13. Antioxid Redox Signal. 2017. PMID: 27488058 Free PMC article.
-
CXCL12-induced macropinocytosis modulates two distinct pathways to activate mTORC1 in macrophages.J Leukoc Biol. 2017 Mar;101(3):683-692. doi: 10.1189/jlb.2A0316-141RR. Epub 2016 Oct 17. J Leukoc Biol. 2017. PMID: 28250113 Free PMC article.
-
Differential ability of proinflammatory and anti-inflammatory macrophages to perform macropinocytosis.Mol Biol Cell. 2018 Jan 1;29(1):53-65. doi: 10.1091/mbc.E17-06-0419. Epub 2017 Nov 1. Mol Biol Cell. 2018. PMID: 29093026 Free PMC article.
-
Macropinocytosis: New Insights Into Its Underappreciated Role in Innate Immune Cell Surveillance.Front Immunol. 2018 Oct 2;9:2286. doi: 10.3389/fimmu.2018.02286. eCollection 2018. Front Immunol. 2018. PMID: 30333835 Free PMC article. Review.
-
Origin, originality, functions, subversions and molecular signalling of macropinocytosis.Int J Med Microbiol. 2002 Feb;291(6-7):487-94. doi: 10.1078/1438-4221-00157. Int J Med Microbiol. 2002. PMID: 11890548 Review.
Cited by
-
Immune complex-induced apoptosis and concurrent immune complex clearance are anti-inflammatory neutrophil functions.Cell Death Dis. 2021 Mar 19;12(4):296. doi: 10.1038/s41419-021-03528-8. Cell Death Dis. 2021. PMID: 33741905 Free PMC article.
-
SLIT2/ROBO1-signaling inhibits macropinocytosis by opposing cortical cytoskeletal remodeling.Nat Commun. 2020 Aug 17;11(1):4112. doi: 10.1038/s41467-020-17651-1. Nat Commun. 2020. PMID: 32807784 Free PMC article.
-
Aluminum hydroxide adjuvant diverts the uptake and trafficking of genetically detoxified pertussis toxin to lysosomes in macrophages.Mol Microbiol. 2022 May;117(5):1173-1195. doi: 10.1111/mmi.14900. Epub 2022 Apr 7. Mol Microbiol. 2022. PMID: 35344242 Free PMC article.
-
The role of the osmosensitive transcription factor NFAT5 in corneal edema resorption after injury.Exp Mol Med. 2023 Mar;55(3):565-573. doi: 10.1038/s12276-023-00954-w. Epub 2023 Mar 3. Exp Mol Med. 2023. PMID: 36869067 Free PMC article.
-
High-throughput Measurement of Dictyostelium discoideum Macropinocytosis by Flow Cytometry.J Vis Exp. 2018 Sep 10;(139):58434. doi: 10.3791/58434. J Vis Exp. 2018. PMID: 30247467 Free PMC article.
References
-
- Kabayama H. et al.. Ca2+ induces macropinocytosis via F-actin depolymerization during growth cone collapse. Mol. Cell Neurosci. 40, 27–38 (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

