IP3 receptors: Take four IP3 to open
- PMID: 27048564
- PMCID: PMC5019202
- DOI: 10.1126/scisignal.aaf6029
IP3 receptors: Take four IP3 to open
Abstract
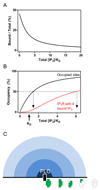
Inositol 1,4,5-trisphosphate receptors (IP3Rs) and ryanodine receptors are the channels responsible for Ca(2+)release from the endoplasmic and sarcoplasmic reticulum. Research inScience Signalingby Alzayadyet al show that all four IP3-binding sites within the tetrameric IP3R must bind IP3before the channel can open, which has important consequences for the distribution of both IP3and IP3R activity within cells.
Copyright © 2016, American Association for the Advancement of Science.
Conflict of interest statement
The authors declare that they have no competing interests.
Figures

Similar articles
-
Defining the stoichiometry of inositol 1,4,5-trisphosphate binding required to initiate Ca2+ release.Sci Signal. 2016 Apr 5;9(422):ra35. doi: 10.1126/scisignal.aad6281. Sci Signal. 2016. PMID: 27048566 Free PMC article.
-
IP3-mediated gating mechanism of the IP3 receptor revealed by mutagenesis and X-ray crystallography.Proc Natl Acad Sci U S A. 2017 May 2;114(18):4661-4666. doi: 10.1073/pnas.1701420114. Epub 2017 Apr 17. Proc Natl Acad Sci U S A. 2017. PMID: 28416699 Free PMC article.
-
IP3 receptors - lessons from analyses ex cellula.J Cell Sci. 2018 Dec 14;132(4):jcs222463. doi: 10.1242/jcs.222463. J Cell Sci. 2018. PMID: 30552138 Review.
-
Structural and functional conservation of the activating Ca2+ binding site in inositol 1,4.5-trisphosphate and ryanodine receptors.Cell Calcium. 2022 Dec;108:102671. doi: 10.1016/j.ceca.2022.102671. Epub 2022 Nov 5. Cell Calcium. 2022. PMID: 36370621
-
Structure and Function of IP3 Receptors.Cold Spring Harb Perspect Biol. 2019 Apr 1;11(4):a035063. doi: 10.1101/cshperspect.a035063. Cold Spring Harb Perspect Biol. 2019. PMID: 30745293 Free PMC article. Review.
Cited by
-
Advances in Intracellular Calcium Signaling Reveal Untapped Targets for Cancer Therapy.Biomedicines. 2021 Aug 24;9(9):1077. doi: 10.3390/biomedicines9091077. Biomedicines. 2021. PMID: 34572262 Free PMC article. Review.
-
Dynamic Ca2+ imaging with a simplified lattice light-sheet microscope: A sideways view of subcellular Ca2+ puffs.Cell Calcium. 2018 May;71:34-44. doi: 10.1016/j.ceca.2017.11.005. Epub 2017 Dec 1. Cell Calcium. 2018. PMID: 29604962 Free PMC article.
-
Inside the Insulin Secretory Granule.Metabolites. 2021 Aug 5;11(8):515. doi: 10.3390/metabo11080515. Metabolites. 2021. PMID: 34436456 Free PMC article. Review.
-
TNFα induces Ca2+ influx to accelerate extrinsic apoptosis in hepatocellular carcinoma cells.J Exp Clin Cancer Res. 2018 Mar 5;37(1):43. doi: 10.1186/s13046-018-0714-6. J Exp Clin Cancer Res. 2018. PMID: 29506556 Free PMC article.
-
Computational investigation of IP3 diffusion.Sci Rep. 2023 Feb 20;13(1):2922. doi: 10.1038/s41598-023-29876-3. Sci Rep. 2023. PMID: 36808161 Free PMC article.
References
-
- Bosanac I, Alattia J-R, Mal TK, Chan J, Talarico S, Tong FK, Tong KI, Yoshikawa F, Furuichi T, Iwai M, Michikawa T, et al. Structure of the inositol 1,4,5-trisphosphate receptor binding core in complex with its ligand. Nature. 2002;420:696–700. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

