Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis
- PMID: 27046227
- PMCID: PMC4822049
- DOI: 10.1038/ncomms11205
Sialylation converts arthritogenic IgG into inhibitors of collagen-induced arthritis
Abstract
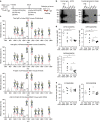
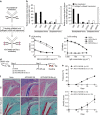
Rheumatoid arthritis (RA)-associated IgG antibodies such as anti-citrullinated protein antibodies (ACPAs) have diverse glycosylation variants; however, key sugar chains modulating the arthritogenic activity of IgG remain to be clarified. Here, we show that reduced sialylation is a common feature of RA-associated IgG in humans and in mouse models of arthritis. Genetically blocking sialylation in activated B cells results in exacerbation of joint inflammation in a collagen-induced arthritis (CIA) model. On the other hand, artificial sialylation of anti-type II collagen antibodies, including ACPAs, not only attenuates arthritogenic activity, but also suppresses the development of CIA in the antibody-infused mice, whereas sialylation of other IgG does not prevent CIA. Thus, our data demonstrate that sialylation levels control the arthritogenicity of RA-associated IgG, presenting a potential target for antigen-specific immunotherapy.
Conflict of interest statement
K.Y. received financial support or fees from AbbVie, Asahi Kasei, Astellas, BMS, Boehringer Ingelheim, Daiichi-Sankyo, ImmunoFuture, Janssen, MitsubishiTanabe, Pfizer, Sanofi, Santen, Takeda, Teijin, Chugai, Eisai, Ono Taisho Toyama and UCB. K.F. received financial support or fees from Astellas, BMS, Chugai, Daiichi-Sankyo, Eisai, Janssen, MitsubishiTanabe, Pfizer, Santen, Takeda, Taisho Toyama and UCB. The remaining authors declare no competing financial interests.
Figures



Similar articles
-
Detection of autoantibodies to citrullinated BiP in rheumatoid arthritis patients and pro-inflammatory role of citrullinated BiP in collagen-induced arthritis.Arthritis Res Ther. 2011;13(6):R191. doi: 10.1186/ar3520. Epub 2011 Nov 22. Arthritis Res Ther. 2011. PMID: 22108001 Free PMC article.
-
Arthritogenic antibodies specific for a major type II collagen triple-helical epitope bind and destabilize cartilage independent of inflammation.Arthritis Rheum. 2008 Jan;58(1):184-96. doi: 10.1002/art.23049. Arthritis Rheum. 2008. PMID: 18163493
-
The role and clinical implications of G6PI in experimental models of rheumatoid arthritis.Arthritis Res Ther. 2005;7(1):20-8. doi: 10.1186/ar1476. Epub 2004 Nov 30. Arthritis Res Ther. 2005. PMID: 15642150 Free PMC article. Review.
-
Epitope-specific recognition of type II collagen by rheumatoid arthritis antibodies is shared with recognition by antibodies that are arthritogenic in collagen-induced arthritis in the mouse.Arthritis Rheum. 2002 Sep;46(9):2339-48. doi: 10.1002/art.10472. Arthritis Rheum. 2002. PMID: 12355481
-
Pathogenic antibody recognition of cartilage.Cell Tissue Res. 2010 Jan;339(1):213-20. doi: 10.1007/s00441-009-0816-8. Epub 2009 Jun 9. Cell Tissue Res. 2010. PMID: 19506910 Review.
Cited by
-
Antibody glycosylation in autoimmune diseases.Autoimmun Rev. 2021 May;20(5):102804. doi: 10.1016/j.autrev.2021.102804. Epub 2021 Mar 14. Autoimmun Rev. 2021. PMID: 33727152 Free PMC article. Review.
-
Synthetic nanobodies as tools to distinguish IgG Fc glycoforms.Proc Natl Acad Sci U S A. 2022 Nov 29;119(48):e2212658119. doi: 10.1073/pnas.2212658119. Epub 2022 Nov 21. Proc Natl Acad Sci U S A. 2022. PMID: 36409896 Free PMC article.
-
Borrelia-specific antibody profiles and complement deposition in joint fluid distinguish antibiotic-refractory from -responsive Lyme arthritis.iScience. 2024 Jan 4;27(2):108804. doi: 10.1016/j.isci.2024.108804. eCollection 2024 Feb 16. iScience. 2024. PMID: 38303696 Free PMC article.
-
Plasma ST6GAL1 regulates IgG sialylation to control IgA nephropathy progression.Ther Adv Chronic Dis. 2021 Oct 11;12:20406223211048644. doi: 10.1177/20406223211048644. eCollection 2021. Ther Adv Chronic Dis. 2021. PMID: 34729155 Free PMC article.
-
Loss of α2-6 sialylation promotes the transformation of synovial fibroblasts into a pro-inflammatory phenotype in arthritis.Nat Commun. 2021 Apr 20;12(1):2343. doi: 10.1038/s41467-021-22365-z. Nat Commun. 2021. PMID: 33879788 Free PMC article.
References
-
- Aletaha D. et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 69, 1580–1588 (2010) . - PubMed
-
- Klareskog L., Ronnelid J., Lundberg K., Padyukov L. & Alfredsson L. Immunity to citrullinated proteins in rheumatoid arthritis. Annu. Rev. Immunol. 26, 651–675 (2008) . - PubMed
-
- Nielen M. M. et al. Specific autoantibodies precede the symptoms of rheumatoid arthritis: a study of serial measurements in blood donors. Arthritis Rheum. 50, 380–386 (2004) . - PubMed
-
- Snir O. et al. Antibodies to several citrullinated antigens are enriched in the joints of rheumatoid arthritis patients. Arthritis Rheum. 62, 44–52 (2010) . - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

