sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance
- PMID: 27042933
- PMCID: PMC4833579
- DOI: 10.1038/nature17392
sFRP2 in the aged microenvironment drives melanoma metastasis and therapy resistance
Abstract
Cancer is a disease of ageing. Clinically, aged cancer patients tend to have a poorer prognosis than young. This may be due to accumulated cellular damage, decreases in adaptive immunity, and chronic inflammation. However, the effects of the aged microenvironment on tumour progression have been largely unexplored. Since dermal fibroblasts can have profound impacts on melanoma progression, we examined whether age-related changes in dermal fibroblasts could drive melanoma metastasis and response to targeted therapy. Here we find that aged fibroblasts secrete a Wnt antagonist, sFRP2, which activates a multi-step signalling cascade in melanoma cells that results in a decrease in β-catenin and microphthalmia-associated transcription factor (MITF), and ultimately the loss of a key redox effector, APE1. Loss of APE1 attenuates the response of melanoma cells to DNA damage induced by reactive oxygen species, rendering the cells more resistant to targeted therapy (vemurafenib). Age-related increases in sFRP2 also augment both angiogenesis and metastasis of melanoma cells. These data provide an integrated view of how fibroblasts in the aged microenvironment contribute to tumour progression, offering new possibilities for the design of therapy for the elderly.
Figures

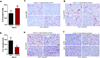




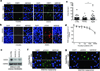
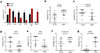
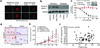




Comment in
-
Frizzled with age: an opportunity for 'gerontological medicine'.Pigment Cell Melanoma Res. 2016 Sep;29(5):488-9. doi: 10.1111/pcmr.12493. Epub 2016 Jun 30. Pigment Cell Melanoma Res. 2016. PMID: 27223582 No abstract available.
-
Melanoma and the Microenvironment--Age Matters.N Engl J Med. 2016 Aug 18;375(7):696-8. doi: 10.1056/NEJMcibr1606907. N Engl J Med. 2016. PMID: 27532838 No abstract available.
Similar articles
-
So You Can Teach Old Fibroblasts New Tricks.Cancer Discov. 2016 Jun;6(6):581-3. doi: 10.1158/2159-8290.CD-16-0503. Cancer Discov. 2016. PMID: 27261482 Free PMC article.
-
Targeting hyperactivation of the AKT survival pathway to overcome therapy resistance of melanoma brain metastases.Cancer Med. 2013 Feb;2(1):76-85. doi: 10.1002/cam4.50. Epub 2013 Feb 3. Cancer Med. 2013. PMID: 24133630 Free PMC article.
-
A Nexus Consisting of Beta-Catenin and Stat3 Attenuates BRAF Inhibitor Efficacy and Mediates Acquired Resistance to Vemurafenib.EBioMedicine. 2016 Jun;8:132-149. doi: 10.1016/j.ebiom.2016.04.037. Epub 2016 May 1. EBioMedicine. 2016. PMID: 27428425 Free PMC article.
-
The discovery of vemurafenib for the treatment of BRAF-mutated metastatic melanoma.Expert Opin Drug Discov. 2016 Sep;11(9):907-16. doi: 10.1080/17460441.2016.1201057. Epub 2016 Jun 23. Expert Opin Drug Discov. 2016. PMID: 27327499 Free PMC article. Review.
-
Vemurafenib in patients with BRAF V600E mutation-positive advanced melanoma.Clin Ther. 2012 Jul;34(7):1474-86. doi: 10.1016/j.clinthera.2012.06.009. Epub 2012 Jun 27. Clin Ther. 2012. PMID: 22742884 Review.
Cited by
-
The dark side of daylight: photoaging and the tumor microenvironment in melanoma progression.J Clin Invest. 2021 Mar 15;131(6):e143763. doi: 10.1172/JCI143763. J Clin Invest. 2021. PMID: 33720046 Free PMC article. Review.
-
Metastasis-Initiating Cells and Ecosystems.Cancer Discov. 2021 Apr;11(4):971-994. doi: 10.1158/2159-8290.CD-21-0010. Cancer Discov. 2021. PMID: 33811127 Free PMC article. Review.
-
Aging-dependent mitochondrial dysfunction mediated by ceramide signaling inhibits antitumor T cell response.Cell Rep. 2021 May 4;35(5):109076. doi: 10.1016/j.celrep.2021.109076. Cell Rep. 2021. PMID: 33951438 Free PMC article.
-
A fibroblast-dependent TGF-β1/sFRP2 noncanonical Wnt signaling axis promotes epithelial metaplasia in idiopathic pulmonary fibrosis.J Clin Invest. 2024 Jul 9;134(18):e174598. doi: 10.1172/JCI174598. J Clin Invest. 2024. PMID: 38980870 Free PMC article. Clinical Trial.
-
Naturally occurring combinations of receptors from single cell transcriptomics in endothelial cells.Sci Rep. 2022 Apr 6;12(1):5807. doi: 10.1038/s41598-022-09616-9. Sci Rep. 2022. PMID: 35388065 Free PMC article.
References
-
- Ruiter D, Bogenrieder T, Elder D, Herlyn M. Melanoma-stroma interactions: structural and functional aspects. Lancet Oncol. 2002;3:35–43. - PubMed
-
- Li G, Satyamoorthy K, Herlyn M. Dynamics of cell interactions and communications during melanoma development. Crit Rev Oral Biol Med. 2002;13:62–70. - PubMed
-
- Hsu MY, Meier F, Herlyn M. Melanoma development and progression: a conspiracy between tumor and host. Differentiation. 2002;70:522–536. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
- P30 CA010815/CA/NCI NIH HHS/United States
- T32 GM007324/GM/NIGMS NIH HHS/United States
- P01 CA114046/CA/NCI NIH HHS/United States
- R01 CA174746/CA/NCI NIH HHS/United States
- R50 CA211199/CA/NCI NIH HHS/United States
- P50 CA174523/CA/NCI NIH HHS/United States
- T32 CA9171-36/CA/NCI NIH HHS/United States
- P01 CA 114046-06/CA/NCI NIH HHS/United States
- ImNIH/Intramural NIH HHS/United States
- R01 CA174746-01/CA/NCI NIH HHS/United States
- P50 CA093372/CA/NCI NIH HHS/United States
- R01 CA196660/CA/NCI NIH HHS/United States
- K12 CA090625/CA/NCI NIH HHS/United States
- K99 CA208012/CA/NCI NIH HHS/United States
- R01 CA160331/CA/NCI NIH HHS/United States
- T32 CA009171/CA/NCI NIH HHS/United States
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

