Using Cryo-EM to Map Small Ligands on Dynamic Metabolic Enzymes: Studies with Glutamate Dehydrogenase
- PMID: 27036132
- PMCID: PMC4885502
- DOI: 10.1124/mol.116.103382
Using Cryo-EM to Map Small Ligands on Dynamic Metabolic Enzymes: Studies with Glutamate Dehydrogenase
Abstract
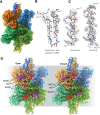
Cryo-electron microscopy (cryo-EM) methods are now being used to determine structures at near-atomic resolution and have great promise in molecular pharmacology, especially in the context of mapping the binding of small-molecule ligands to protein complexes that display conformational flexibility. We illustrate this here using glutamate dehydrogenase (GDH), a 336-kDa metabolic enzyme that catalyzes the oxidative deamination of glutamate. Dysregulation of GDH leads to a variety of metabolic and neurologic disorders. Here, we report near-atomic resolution cryo-EM structures, at resolutions ranging from 3.2 Å to 3.6 Å for GDH complexes, including complexes for which crystal structures are not available. We show that the binding of the coenzyme NADH alone or in concert with GTP results in a binary mixture in which the enzyme is in either an "open" or "closed" state. Whereas the structure of NADH in the active site is similar between the open and closed states, it is unexpectedly different at the regulatory site. Our studies thus demonstrate that even in instances when there is considerable structural information available from X-ray crystallography, cryo-EM methods can provide useful complementary insights into regulatory mechanisms for dynamic protein complexes.
Copyright © 2016 U.S. Government work not protected by U.S. copyright.
Figures



Similar articles
-
Structures of bovine glutamate dehydrogenase complexes elucidate the mechanism of purine regulation.J Mol Biol. 2001 Mar 23;307(2):707-20. doi: 10.1006/jmbi.2001.4499. J Mol Biol. 2001. PMID: 11254391
-
Breaking Cryo-EM Resolution Barriers to Facilitate Drug Discovery.Cell. 2016 Jun 16;165(7):1698-1707. doi: 10.1016/j.cell.2016.05.040. Epub 2016 May 26. Cell. 2016. PMID: 27238019 Free PMC article.
-
Allosteric regulation of glutamate dehydrogenase deamination activity.Sci Rep. 2020 Oct 5;10(1):16523. doi: 10.1038/s41598-020-73743-4. Sci Rep. 2020. PMID: 33020580 Free PMC article.
-
Sample preparation of biological macromolecular assemblies for the determination of high-resolution structures by cryo-electron microscopy.Microscopy (Oxf). 2016 Feb;65(1):23-34. doi: 10.1093/jmicro/dfv367. Epub 2015 Dec 15. Microscopy (Oxf). 2016. PMID: 26671943 Review.
-
Resolution advances in cryo-EM enable application to drug discovery.Curr Opin Struct Biol. 2016 Dec;41:194-202. doi: 10.1016/j.sbi.2016.07.009. Epub 2016 Aug 20. Curr Opin Struct Biol. 2016. PMID: 27552081 Free PMC article. Review.
Cited by
-
Dataset from a human-in-the-loop approach to identify functionally important protein residues from literature.Sci Data. 2024 Sep 27;11(1):1032. doi: 10.1038/s41597-024-03841-9. Sci Data. 2024. PMID: 39333508 Free PMC article.
-
A comparative analysis of fruit fly and human glutamate dehydrogenases in Drosophila melanogaster sperm development.Front Cell Dev Biol. 2023 Nov 2;11:1281487. doi: 10.3389/fcell.2023.1281487. eCollection 2023. Front Cell Dev Biol. 2023. PMID: 38020911 Free PMC article.
-
Technologies for Direct Detection of Covalent Protein-Drug Adducts.Pharmaceuticals (Basel). 2023 Apr 5;16(4):547. doi: 10.3390/ph16040547. Pharmaceuticals (Basel). 2023. PMID: 37111304 Free PMC article. Review.
-
The Knowns and Unknowns in Protein-Metabolite Interactions.Int J Mol Sci. 2023 Feb 19;24(4):4155. doi: 10.3390/ijms24044155. Int J Mol Sci. 2023. PMID: 36835565 Free PMC article. Review.
-
The Mitochondrial Protein MitoNEET as a Probe for the Allostery of Glutamate Dehydrogenase.Molecules. 2022 Nov 29;27(23):8314. doi: 10.3390/molecules27238314. Molecules. 2022. PMID: 36500407 Free PMC article.
References
-
- Allen A, Kwagh J, Fang J, Stanley CA, Smith TJ. (2004) Evolution of glutamate dehydrogenase regulation of insulin homeostasis is an example of molecular exaptation. Biochemistry 43:14431–14443. - PubMed
-
- Bailey J, Bell ET, Bell JE. (1982) Regulation of bovine glutamate dehydrogenase. The effects of pH and ADP. J Biol Chem 257:5579–5583. - PubMed
-
- Banerjee S, Schmidt T, Fang J, Stanley CA, Smith TJ. (2003) Structural studies on ADP activation of mammalian glutamate dehydrogenase and the evolution of regulation. Biochemistry 42:3446–3456. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
