Overview of Alzheimer's Disease and Some Therapeutic Approaches Targeting Aβ by Using Several Synthetic and Herbal Compounds
- PMID: 27034741
- PMCID: PMC4807045
- DOI: 10.1155/2016/7361613
Overview of Alzheimer's Disease and Some Therapeutic Approaches Targeting Aβ by Using Several Synthetic and Herbal Compounds
Abstract
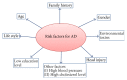
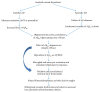
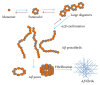
Alzheimer's disease (AD) is a complex age-related neurodegenerative disease. In this review, we carefully detail amyloid-β metabolism and its role in AD. We also consider the various genetic animal models used to evaluate therapeutics. Finally, we consider the role of synthetic and plant-based compounds in therapeutics.
Figures
Similar articles
-
Jowiseungchungtang Inhibits Amyloid-β Aggregation and Amyloid-β-Mediated Pathology in 5XFAD Mice.Int J Mol Sci. 2018 Dec 13;19(12):4026. doi: 10.3390/ijms19124026. Int J Mol Sci. 2018. PMID: 30551564 Free PMC article.
-
Natural Medicines and Their Underlying Mechanisms of Prevention and Recovery from Amyloid Β-Induced Axonal Degeneration in Alzheimer's Disease.Int J Mol Sci. 2020 Jun 30;21(13):4665. doi: 10.3390/ijms21134665. Int J Mol Sci. 2020. PMID: 32630004 Free PMC article. Review.
-
Exploring Aβ Proteotoxicity and Therapeutic Candidates Using Drosophila melanogaster.Int J Mol Sci. 2021 Sep 28;22(19):10448. doi: 10.3390/ijms221910448. Int J Mol Sci. 2021. PMID: 34638786 Free PMC article. Review.
-
Are N- and C-terminally truncated Aβ species key pathological triggers in Alzheimer's disease?J Biol Chem. 2018 Oct 5;293(40):15419-15428. doi: 10.1074/jbc.R118.003999. Epub 2018 Aug 24. J Biol Chem. 2018. PMID: 30143530 Free PMC article. Review.
-
Genetic Dissection of Alzheimer's Disease Using Drosophila Models.Int J Mol Sci. 2020 Jan 30;21(3):884. doi: 10.3390/ijms21030884. Int J Mol Sci. 2020. PMID: 32019113 Free PMC article. Review.
Cited by
-
Human CALHM5: Insight in large pore lipid gating ATP channel and associated neurological pathologies.Mol Cell Biochem. 2021 Oct;476(10):3711-3718. doi: 10.1007/s11010-021-04198-y. Epub 2021 Jun 5. Mol Cell Biochem. 2021. PMID: 34089472 Review.
-
Lupeol, a Plant-Derived Triterpenoid, Protects Mice Brains against Aβ-Induced Oxidative Stress and Neurodegeneration.Biomedicines. 2020 Sep 26;8(10):380. doi: 10.3390/biomedicines8100380. Biomedicines. 2020. PMID: 32993092 Free PMC article.
-
Dissecting Sex-Related Cognition between Alzheimer's Disease and Diabetes: From Molecular Mechanisms to Potential Therapeutic Strategies.Oxid Med Cell Longev. 2021 Mar 5;2021:4572471. doi: 10.1155/2021/4572471. eCollection 2021. Oxid Med Cell Longev. 2021. PMID: 33747345 Free PMC article. Review.
-
Activation of Microbiota Sensing - Free Fatty Acid Receptor 2 Signaling Ameliorates Amyloid-β Induced Neurotoxicity by Modulating Proteolysis-Senescence Axis.Front Aging Neurosci. 2021 Oct 5;13:735933. doi: 10.3389/fnagi.2021.735933. eCollection 2021. Front Aging Neurosci. 2021. PMID: 34707491 Free PMC article.
-
Mixed Medicinal Mushroom Mycelia Attenuates Alzheimer's Disease Pathologies In Vitro and In Vivo.Curr Issues Mol Biol. 2023 Aug 15;45(8):6775-6789. doi: 10.3390/cimb45080428. Curr Issues Mol Biol. 2023. PMID: 37623247 Free PMC article.
References
-
- Selkoe D. J. Alzheimer's disease: genes, proteins, and therapy. Physiological Reviews. 2001;81(2):741–766. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

