Blood-brain barrier breakdown promotes macrophage infiltration and cognitive impairment in leptin receptor-deficient mice
- PMID: 27034250
- PMCID: PMC5363667
- DOI: 10.1177/0271678X16642233
Blood-brain barrier breakdown promotes macrophage infiltration and cognitive impairment in leptin receptor-deficient mice
Abstract
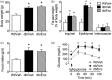
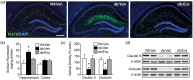
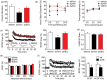
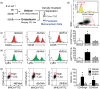
Accumulating evidence indicates that obesity accelerates the onset of cognitive decline. While mechanisms are still being identified, obesity promotes peripheral inflammation and increases blood-brain barrier (BBB) permeability. However, no studies have manipulated vascular permeability in obesity to determine whether BBB breakdown underlies memory deficits. Protein kinase Cβ (PKCβ) activation destabilizes the BBB, and we used a PKCβ inhibitor (Enzastaurin) to block BBB leakiness in leptin receptor-deficient (db/db) mice. Enzastaurin reversed BBB breakdown in db/db mice and normalized hippocampal function without affecting obesity or metabolism. Flow cytometric analysis of forebrain mononuclear cells (FMCs) from db/db mice revealed macrophage infiltration and induction of the activation marker MHCII in microglia and macrophages. Enzastaurin eliminated macrophage infiltration and MHCII induction, and protein array profiling revealed parallel reductions in IL1β, IL6, MCP1, and TNFα. To investigate whether these signals attract peripheral monocytes, FMCs from Wt and db/db mice were plated below migration inserts containing peritoneal macrophages. Peritoneal macrophages from db/db mice exhibit increases in transmigration that were blocked by recombinant IL1RA. These studies indicate that BBB breakdown impairs cognition in obesity and diabetes by allowing macrophage infiltration, with a potential role for IL1β in trafficking of peripheral monocytes into the brain.
Keywords: Diabetes; hippocampus; inflammation; learning and memory; microglia; obesity.
© The Author(s) 2016.
Figures

Similar articles
-
Endothelial Adora2a Activation Promotes Blood-Brain Barrier Breakdown and Cognitive Impairment in Mice with Diet-Induced Insulin Resistance.J Neurosci. 2019 May 22;39(21):4179-4192. doi: 10.1523/JNEUROSCI.2506-18.2019. Epub 2019 Mar 18. J Neurosci. 2019. PMID: 30886019 Free PMC article.
-
Antidiabetic drugs restore abnormal transport of amyloid-β across the blood-brain barrier and memory impairment in db/db mice.Neuropharmacology. 2016 Feb;101:123-36. doi: 10.1016/j.neuropharm.2015.07.023. Epub 2015 Jul 26. Neuropharmacology. 2016. PMID: 26211973
-
HDAC3 inhibition prevents blood-brain barrier permeability through Nrf2 activation in type 2 diabetes male mice.J Neuroinflammation. 2019 May 17;16(1):103. doi: 10.1186/s12974-019-1495-3. J Neuroinflammation. 2019. PMID: 31101061 Free PMC article.
-
Deficient Leptin Cellular Signaling Plays a Key Role in Brain Ultrastructural Remodeling in Obesity and Type 2 Diabetes Mellitus.Int J Mol Sci. 2021 May 21;22(11):5427. doi: 10.3390/ijms22115427. Int J Mol Sci. 2021. PMID: 34063911 Free PMC article. Review.
-
The blood-brain barrier as a cause of obesity.Curr Pharm Des. 2008;14(16):1606-14. doi: 10.2174/138161208784705496. Curr Pharm Des. 2008. PMID: 18673202 Review.
Cited by
-
Three-dimensional remodeling of functional cerebrovascular architecture and gliovascular unit in leptin receptor-deficient mice.J Cereb Blood Flow Metab. 2021 Jul;41(7):1547-1562. doi: 10.1177/0271678X211006596. Epub 2021 Apr 4. J Cereb Blood Flow Metab. 2021. PMID: 33818188 Free PMC article.
-
Tirzepatide ameliorates spatial learning and memory impairment through modulation of aberrant insulin resistance and inflammation response in diabetic rats.Front Pharmacol. 2023 Aug 28;14:1146960. doi: 10.3389/fphar.2023.1146960. eCollection 2023. Front Pharmacol. 2023. PMID: 37701028 Free PMC article.
-
Visceral adipose NLRP3 impairs cognition in obesity via IL-1R1 on CX3CR1+ cells.J Clin Invest. 2020 Apr 1;130(4):1961-1976. doi: 10.1172/JCI126078. J Clin Invest. 2020. PMID: 31935195 Free PMC article.
-
Palmitic Acid and Oleic Acid Differently Modulate TLR2-Mediated Inflammatory Responses in Microglia and Macrophages.Mol Neurobiol. 2022 Apr;59(4):2348-2362. doi: 10.1007/s12035-022-02756-z. Epub 2022 Jan 25. Mol Neurobiol. 2022. PMID: 35079937 Free PMC article.
-
Young blood-mediated cerebromicrovascular rejuvenation through heterochronic parabiosis: enhancing blood-brain barrier integrity and capillarization in the aged mouse brain.Geroscience. 2024 Oct;46(5):4415-4442. doi: 10.1007/s11357-024-01154-8. Epub 2024 May 10. Geroscience. 2024. PMID: 38727872 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

