Habenula cholinergic neurons regulate anxiety during nicotine withdrawal via nicotinic acetylcholine receptors
- PMID: 27020042
- PMCID: PMC4982553
- DOI: 10.1016/j.neuropharm.2016.03.039
Habenula cholinergic neurons regulate anxiety during nicotine withdrawal via nicotinic acetylcholine receptors
Abstract
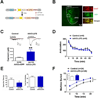
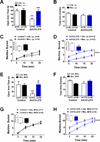
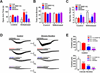
Cholinergic neurons in the medial habenula (MHb) modulate anxiety during nicotine withdrawal although the molecular neuroadaptation(s) within the MHb that induce affective behaviors during nicotine cessation is largely unknown. MHb cholinergic neurons are unique in that they robustly express neuronal nicotinic acetylcholine receptors (nAChRs), although their behavioral role as autoreceptors in these neurons has not been described. To test the hypothesis that nAChR signaling in MHb cholinergic neurons could modulate anxiety, we expressed novel "gain of function" nAChR subunits selectively in MHb cholinergic neurons of adult mice. Mice expressing these mutant nAChRs exhibited increased anxiety-like behavior that was alleviated by blockade with a nAChR antagonist. To test the hypothesis that anxiety induced by nicotine withdrawal may be mediated by increased MHb nicotinic receptor signaling, we infused nAChR subtype selective antagonists into the MHb of nicotine naïve and withdrawn mice. While antagonists had little effect on nicotine naïve mice, blocking α4β2 or α6β2, but not α3β4 nAChRs in the MHb alleviated anxiety in mice undergoing nicotine withdrawal. Consistent with behavioral results, there was increased functional expression of nAChRs containing the α6 subunit in MHb neurons that also expressed the α4 subunit. Together, these data indicate that MHb cholinergic neurons regulate nicotine withdrawal-induced anxiety via increased signaling through nicotinic receptors containing the α6 subunit and point toward nAChRs in MHb cholinergic neurons as molecular targets for smoking cessation therapeutics.
Keywords: Acetylcholine; Anxiety; Habenula; Nicotine; Withdrawal.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures

Similar articles
-
Nicotine Dependence Reveals Distinct Responses from Neurons and Their Resident Nicotinic Receptors in Medial Habenula.Mol Pharmacol. 2015 Dec;88(6):1035-44. doi: 10.1124/mol.115.101444. Epub 2015 Oct 1. Mol Pharmacol. 2015. PMID: 26429939 Free PMC article.
-
Chronic Nicotine Exposure Alters the Neurophysiology of Habenulo-Interpeduncular Circuitry.J Neurosci. 2019 May 29;39(22):4268-4281. doi: 10.1523/JNEUROSCI.2816-18.2019. Epub 2019 Mar 13. J Neurosci. 2019. PMID: 30867261 Free PMC article.
-
Reexposure to nicotine during withdrawal increases the pacemaking activity of cholinergic habenular neurons.Proc Natl Acad Sci U S A. 2013 Oct 15;110(42):17077-82. doi: 10.1073/pnas.1313103110. Epub 2013 Sep 30. Proc Natl Acad Sci U S A. 2013. PMID: 24082085 Free PMC article.
-
The habenulo-interpeduncular pathway in nicotine aversion and withdrawal.Neuropharmacology. 2015 Sep;96(Pt B):213-22. doi: 10.1016/j.neuropharm.2014.11.019. Epub 2014 Dec 2. Neuropharmacology. 2015. PMID: 25476971 Free PMC article. Review.
-
Neural circuits and nicotinic acetylcholine receptors mediate the cholinergic regulation of midbrain dopaminergic neurons and nicotine dependence.Acta Pharmacol Sin. 2020 Jan;41(1):1-9. doi: 10.1038/s41401-019-0299-4. Epub 2019 Sep 25. Acta Pharmacol Sin. 2020. PMID: 31554960 Free PMC article. Review.
Cited by
-
α-Conotoxin TxIB Improved Behavioral Abnormality and Changed Gene Expression in Zebrafish (Danio rerio) Induced by Alcohol Withdrawal.Front Pharmacol. 2022 Feb 1;13:802917. doi: 10.3389/fphar.2022.802917. eCollection 2022. Front Pharmacol. 2022. PMID: 35177988 Free PMC article.
-
Developmental Changes in Habenular and Striatal Social Reinforcement Responsivity Across Adolescence Linked With Substance Use.Biol Psychiatry. 2023 Dec 1;94(11):888-897. doi: 10.1016/j.biopsych.2023.04.018. Epub 2023 Apr 28. Biol Psychiatry. 2023. PMID: 37120062 Free PMC article.
-
Cell-type-specific population dynamics of diverse reward computations.Cell. 2022 Sep 15;185(19):3568-3587.e27. doi: 10.1016/j.cell.2022.08.019. Cell. 2022. PMID: 36113428 Free PMC article.
-
Midbrain circuits of novelty processing.Neurobiol Learn Mem. 2020 Dec;176:107323. doi: 10.1016/j.nlm.2020.107323. Epub 2020 Oct 11. Neurobiol Learn Mem. 2020. PMID: 33053429 Free PMC article. Review.
-
Nicotine regulates activity of lateral habenula neurons via presynaptic and postsynaptic mechanisms.Sci Rep. 2016 Sep 6;6:32937. doi: 10.1038/srep32937. Sci Rep. 2016. PMID: 27596561 Free PMC article.
References
-
- Benowitz NL. Neurobiology of nicotine addiction: implications for smoking cessation treatment. Am J Med. 2008;121:S3–S10. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

