Classical dendritic cells are required for dietary antigen-mediated induction of peripheral T(reg) cells and tolerance
- PMID: 27019226
- PMCID: PMC4837106
- DOI: 10.1038/ni.3408
Classical dendritic cells are required for dietary antigen-mediated induction of peripheral T(reg) cells and tolerance
Abstract
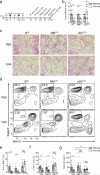
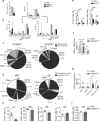
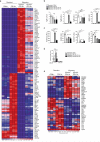
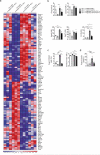
Oral tolerance prevents pathological inflammatory responses to innocuous foreign antigens by peripheral regulatory T cells (pT(reg) cells). However, whether a particular subset of antigen-presenting cells (APCs) is required during dietary antigen exposure for the 'instruction' of naive CD4(+) T cells to differentiate into pT(reg) cells has not been defined. Using myeloid lineage-specific APC depletion in mice, we found that monocyte-derived APCs were dispensable, while classical dendritic cells (cDCs) were critical, for pT(reg) cell induction and oral tolerance. CD11b(-) cDCs from the gut-draining lymph nodes efficiently induced pT(reg) cells and, conversely, loss of transcription factor IRF8-dependent CD11b(-) cDCs impaired their polarization, although oral tolerance remained intact. These data reveal the hierarchy of cDC subsets in the induction of pT(reg) cells and their redundancy during the development of oral tolerance.
Figures

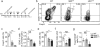

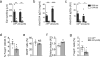
Comment in
-
A dendritic cell subset designed for oral tolerance.Nat Immunol. 2016 May;17(5):474-6. doi: 10.1038/ni.3435. Nat Immunol. 2016. PMID: 27092796 No abstract available.
Similar articles
-
Stroma-dependent development of two dendritic-like cell types with distinct antigen presenting capability.Exp Hematol. 2013 Mar;41(3):281-92. doi: 10.1016/j.exphem.2012.11.003. Epub 2012 Nov 23. Exp Hematol. 2013. PMID: 23178375
-
Oral CD103-CD11b+ classical dendritic cells present sublingual antigen and induce Foxp3+ regulatory T cells in draining lymph nodes.Mucosal Immunol. 2017 Jan;10(1):79-90. doi: 10.1038/mi.2016.46. Epub 2016 May 11. Mucosal Immunol. 2017. PMID: 27166558
-
Functional specializations of intestinal dendritic cell and macrophage subsets that control Th17 and regulatory T cell responses are dependent on the T cell/APC ratio, source of mouse strain, and regional localization.J Immunol. 2011 Jul 15;187(2):733-47. doi: 10.4049/jimmunol.1002701. Epub 2011 Jun 10. J Immunol. 2011. PMID: 21666057 Free PMC article.
-
The impact of cell-bound antigen transport on mucosal tolerance induction.J Leukoc Biol. 2007 Oct;82(4):795-800. doi: 10.1189/jlb.0307144. Epub 2007 Jun 12. J Leukoc Biol. 2007. PMID: 17565048 Review.
-
Induction of Immune Tolerance to Dietary Antigens.Adv Exp Med Biol. 2015;850:93-118. doi: 10.1007/978-3-319-15774-0_8. Adv Exp Med Biol. 2015. PMID: 26324349 Review.
Cited by
-
RelB Deficiency in Dendritic Cells Protects from Autoimmune Inflammation Due to Spontaneous Accumulation of Tissue T Regulatory Cells.J Immunol. 2019 Nov 15;203(10):2602-2613. doi: 10.4049/jimmunol.1801530. Epub 2019 Oct 2. J Immunol. 2019. PMID: 31578269 Free PMC article.
-
Silencing EPB41 Gene Expression Leads to Cell Cycle Arrest, Migration Inhibition, and Upregulation of Cell Surface Antigen in DC2.4 Cells.Med Sci Monit. 2020 Mar 11;26:e920594. doi: 10.12659/MSM.920594. Med Sci Monit. 2020. PMID: 32157074 Free PMC article.
-
Molecular regulation of dendritic cell development and function in homeostasis, inflammation, and cancer.Mol Immunol. 2019 Jun;110:24-39. doi: 10.1016/j.molimm.2018.01.014. Epub 2018 Mar 15. Mol Immunol. 2019. PMID: 29549977 Free PMC article. Review.
-
PD-L1+ and XCR1+ dendritic cells are region-specific regulators of gut homeostasis.Nat Commun. 2021 Aug 13;12(1):4907. doi: 10.1038/s41467-021-25115-3. Nat Commun. 2021. PMID: 34389726 Free PMC article.
-
Distal Immunization and Systemic Cytokines Establish a Transient Immune Alert State in the Intestine.J Immunol. 2024 Aug 1;213(3):373-383. doi: 10.4049/jimmunol.2400209. J Immunol. 2024. PMID: 38884660
References
-
- Kretschmer K, Apostolou I, Hawiger D, Khazaie K, Nussenzweig MC, von Boehmer H. Inducing and expanding regulatory T cell populations by foreign antigen. Nat Immunol. 2005;6(12):1219–1227. - PubMed
-
- Curotto de Lafaille MA, Kutchukhidze N, Shen S, Ding Y, Yee H, Lafaille JJ. Adaptive Foxp3+ regulatory T cell-dependent and -independent control of allergic inflammation. Immunity. 2008;29(1):114–126. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

