Modeling menopause: The utility of rodents in translational behavioral endocrinology research
- PMID: 27013283
- PMCID: PMC4829404
- DOI: 10.1016/j.maturitas.2016.01.015
Modeling menopause: The utility of rodents in translational behavioral endocrinology research
Abstract
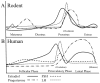
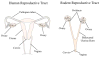
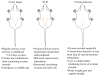
The human menopause transition and aging are each associated with an increase in a variety of health risk factors including, but not limited to, cardiovascular disease, osteoporosis, cancer, diabetes, stroke, sexual dysfunction, affective disorders, sleep disturbances, and cognitive decline. It is challenging to systematically evaluate the biological underpinnings associated with the menopause transition in the human population. For this reason, rodent models have been invaluable tools for studying the impact of gonadal hormone fluctuations and eventual decline on a variety of body systems. While it is essential to keep in mind that some of the mechanisms associated with aging and the transition into a reproductively senescent state can differ when translating from one species to another, animal models provide researchers with opportunities to gain a fundamental understanding of the key elements underlying reproduction and aging processes, paving the way to explore novel pathways for intervention associated with known health risks. Here, we discuss the utility of several rodent models used in the laboratory for translational menopause research, examining the benefits and drawbacks in helping us to better understand aging and the menopause transition in women. The rodent models discussed are ovary-intact, ovariectomy, and 4-vinylcylohexene diepoxide for the menopause transition. We then describe how these models may be implemented in the laboratory, particularly in the context of cognition. Ultimately, we aim to use these animal models to elucidate novel perspectives and interventions for maintaining a high quality of life in women, and to potentially prevent or postpone the onset of negative health consequences associated with these significant life changes during aging.
Keywords: Aging; Female; Hormones; Menopause; Model; Rodent.
Copyright © 2016 Elsevier Ireland Ltd. All rights reserved.
Conflict of interest statement
I, Heather A. Bimonte-Nelson, have no conflicts of interest to declare.
I, Stephanie V. Koebele, have no conflicts of interest to declare.
Figures




Similar articles
-
Nonhuman primate models of menopause workshop.Biol Reprod. 2003 Jan;68(1):10-8. doi: 10.1095/biolreprod.102.005215. Biol Reprod. 2003. PMID: 12493689
-
Recent female mouse models displaying advanced reproductive aging.Exp Gerontol. 2006 Feb;41(2):117-22. doi: 10.1016/j.exger.2005.10.010. Epub 2005 Dec 13. Exp Gerontol. 2006. PMID: 16352410 Review.
-
Cognitive changes across the menopause transition: A longitudinal evaluation of the impact of age and ovarian status on spatial memory.Horm Behav. 2017 Jan;87:96-114. doi: 10.1016/j.yhbeh.2016.10.010. Epub 2016 Oct 26. Horm Behav. 2017. PMID: 27793768 Free PMC article.
-
Translational cognitive endocrinology: designing rodent experiments with the goal to ultimately enhance cognitive health in women.Brain Res. 2013 Jun 13;1514:50-62. doi: 10.1016/j.brainres.2013.01.020. Epub 2013 Feb 4. Brain Res. 2013. PMID: 23391594 Free PMC article. Review.
-
Role of Ovarian Hormones in the Modulation of Sleep in Females Across the Adult Lifespan.Endocrinology. 2020 Sep 1;161(9):bqaa128. doi: 10.1210/endocr/bqaa128. Endocrinology. 2020. PMID: 32735650 Free PMC article. Review.
Cited by
-
Natriuretic Peptides-New Targets for Neurocontrol of Blood Pressure via Baroreflex Afferent Pathway.Int J Mol Sci. 2022 Nov 7;23(21):13619. doi: 10.3390/ijms232113619. Int J Mol Sci. 2022. PMID: 36362405 Free PMC article.
-
Evaluating the Cognitive Impacts of Drospirenone, a Spironolactone-Derived Progestin, Independently and in Combination With Ethinyl Estradiol in Ovariectomized Adult Rats.Front Neurosci. 2022 May 25;16:885321. doi: 10.3389/fnins.2022.885321. eCollection 2022. Front Neurosci. 2022. PMID: 35692432 Free PMC article.
-
Pre-Clinical Models in Implant Dentistry: Past, Present, Future.Biomedicines. 2021 Oct 26;9(11):1538. doi: 10.3390/biomedicines9111538. Biomedicines. 2021. PMID: 34829765 Free PMC article. Review.
-
Sex hormones and stroke: Beyond estrogens.Horm Behav. 2019 May;111:87-95. doi: 10.1016/j.yhbeh.2018.10.010. Epub 2018 Nov 20. Horm Behav. 2019. PMID: 30713101 Free PMC article. Review.
-
Murine Orchiectomy and Ovariectomy to Reduce Sex Hormone Production.J Vis Exp. 2023 Nov 17;(201):10.3791/64379. doi: 10.3791/64379. J Vis Exp. 2023. PMID: 38047564 Free PMC article.
References
-
- North American Menopause Society. Menopause Practice A Clinician’s Guide. 5. 2014.
-
- Hoffman BL, Schorge JO, Schaffer JI, Halvorson LM, Bradshaw KD, Cunningham FG, Calver LE. In: Williams Gynecology. 2. Fried A, Boyle PJ, editors. McGraw-Hill; New York: 2012.
-
- Al-Safi Z, Santoro N. Menopausal hormone therapy and menopausal symptoms. Fertil Steril. 2014;101:905–915. - PubMed
-
- Hale G, Robertson D, Burger H. The perimenopausal woman: endocrinology and management. J Steroid Biochem Mol Biol. 2014;142:121–131. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

