Enhanced local and systemic anti-melanoma CD8+ T cell responses after memory T cell-based adoptive immunotherapy in mice
- PMID: 27011014
- PMCID: PMC4857203
- DOI: 10.1007/s00262-016-1823-8
Enhanced local and systemic anti-melanoma CD8+ T cell responses after memory T cell-based adoptive immunotherapy in mice
Abstract
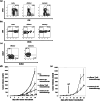
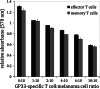
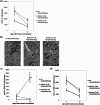
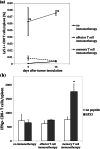
Adoptive cell transfer (ACT) melanoma immunotherapy typically employs acutely activated effector CD8+ T cells for their ability to rapidly recognize and clear antigen. We have previously observed that effector CD8+ T cells are highly susceptible to melanoma-induced suppression, whereas memory CD8+ T cells are not. Although memory T cells have been presumed to be potentially advantageous for ACT, the kinetics of local and systemic T cell responses after effector and memory ACT have not been compared. B16F10 melanoma cells stably transfected to express very low levels of the lymphocytic choriomeningitis virus (LCMV) peptide GP33 (B16GP33) were inoculated into syngeneic C57BL/6 mice. Equal numbers of bona fide naïve, effector, or memory phenotype GP33-specific CD8+ T cells were adoptively transferred into mice 1 day after B16GP33 inoculation. The efficacy of ACT immunotherapy was kinetically assessed using serial tumor measurements and flow cytometric analyses of local and systemic CD8+ T cell responses. Control of B16GP33 tumor growth, persistence of adoptively transferred CD8+ cells, intratumoral infiltration of CD8+ T cells, and systemic CD8+ T cell responsiveness to GP33 were strongest after ACT of memory CD8+ T cells. Following surgical tumor resection and melanoma tumor challenge, only mice receiving memory T cell-based ACT immunotherapy exhibited durable tumor-specific immunity. These findings demonstrate how the use of non-expanded memory CD8+ T cells may enhance ACT immunotherapeutic efficacy.
Keywords: Adoptive transfer; Effector; Immunotherapy; Melanoma; Memory; T cell.
Conflict of interest statement
All authors have declared that there are no financial conflicts of interest with regard to this work.
Figures

Similar articles
-
Ctla-4 blockade plus adoptive T-cell transfer promotes optimal melanoma immunity in mice.J Immunother. 2015 Feb-Mar;38(2):54-61. doi: 10.1097/CJI.0000000000000064. J Immunother. 2015. PMID: 25658614 Free PMC article.
-
Co-transfer of tumor-specific effector and memory CD8+ T cells enhances the efficacy of adoptive melanoma immunotherapy in a mouse model.J Immunother Cancer. 2018 May 29;6(1):41. doi: 10.1186/s40425-018-0358-2. J Immunother Cancer. 2018. PMID: 29843822 Free PMC article.
-
Melanoma-induced suppression of tumor antigen-specific T cell expansion is comparable to suppression of global T cell expansion.Cell Immunol. 2011;271(1):104-9. doi: 10.1016/j.cellimm.2011.06.011. Epub 2011 Jun 17. Cell Immunol. 2011. PMID: 21741629 Free PMC article.
-
Role of memory T cell subsets for adoptive immunotherapy.Semin Immunol. 2016 Feb;28(1):28-34. doi: 10.1016/j.smim.2016.02.001. Epub 2016 Mar 11. Semin Immunol. 2016. PMID: 26976826 Free PMC article. Review.
-
[Cancer immunotherapy and immunological memory].Nihon Rinsho Meneki Gakkai Kaishi. 2016;39(1):18-22. doi: 10.2177/jsci.39.18. Nihon Rinsho Meneki Gakkai Kaishi. 2016. PMID: 27181230 Review. Japanese.
Cited by
-
Early memory differentiation and cell death resistance in T cells predicts melanoma response to sequential anti-CTLA4 and anti-PD1 immunotherapy.Genes Immun. 2021 Jun;22(2):108-119. doi: 10.1038/s41435-021-00138-4. Epub 2021 Jun 2. Genes Immun. 2021. PMID: 34079092
-
Non-thermal histotripsy tumor ablation promotes abscopal immune responses that enhance cancer immunotherapy.J Immunother Cancer. 2020 Jan;8(1):e000200. doi: 10.1136/jitc-2019-000200. J Immunother Cancer. 2020. PMID: 31940590 Free PMC article.
-
Moderate physical exercise improves lymphocyte function in melanoma-bearing mice on a high-fat diet.Nutr Metab (Lond). 2019 Sep 12;16:63. doi: 10.1186/s12986-019-0394-z. eCollection 2019. Nutr Metab (Lond). 2019. PMID: 31528182 Free PMC article.
-
Type I IFN blockade uncouples immunotherapy-induced antitumor immunity and autoimmune toxicity.J Clin Invest. 2019 Feb 1;129(2):518-530. doi: 10.1172/JCI121004. Epub 2018 Dec 18. J Clin Invest. 2019. PMID: 30422820 Free PMC article.
-
Escherichia coli adhesin protein-conjugated thermal responsive hybrid nanoparticles for photothermal and immunotherapy against cancer and its metastasis.J Immunother Cancer. 2021 Jul;9(7):e002666. doi: 10.1136/jitc-2021-002666. J Immunother Cancer. 2021. PMID: 34230112 Free PMC article.
References
-
- Rosenberg SA, Yannelli JR, Yang JC, Topalian SL, Schwartzentruber DJ, Weber JS, Parkinson DR, Seipp CA, Einhorn JH, White DE. Treatment of patients with metastatic melanoma with autologous tumor-infiltrating lymphocytes and interleukin 2. J Natl Cancer Inst. 1994;86:1159–1166. doi: 10.1093/jnci/86.15.1159. - DOI - PubMed
-
- Rosenberg SA, Yang JC, Sherry RM, Kammula UW, Hughes MS, Phan GQ, Citrin DE, Restifo NP, Robbins PF, Wunderlich JR, Morton KE, Laurencot CM, Steinberg SM, White DE, Dudley ME. Durable complete responses in heavily pretreated patients with metastatic melanoma using T-cell transfer immunotherapy. Clin Cancer Res. 2011;17:4550–4557. doi: 10.1158/1078-0432.CCR-11-0116. - DOI - PMC - PubMed
-
- Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–2357. doi: 10.1200/JCO.2005.00.240. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

