Characterization of outbreak response strategies and potential vaccine stockpile needs for the polio endgame
- PMID: 27009272
- PMCID: PMC4806487
- DOI: 10.1186/s12879-016-1465-7
Characterization of outbreak response strategies and potential vaccine stockpile needs for the polio endgame
Abstract
Background: Following successful eradication of wild polioviruses and planned globally-coordinated cessation of oral poliovirus vaccine (OPV), national and global health leaders may need to respond to outbreaks from reintroduced live polioviruses, particularly vaccine-derived polioviruses (VDPVs). Preparing outbreak response plans and assessing potential vaccine needs from an emergency stockpile require consideration of the different national risks and conditions as they change with time after OPV cessation.
Methods: We used an integrated global model to consider several key issues related to managing poliovirus risks and outbreak response, including the time interval during which monovalent OPV (mOPV) can be safely used following homotypic OPV cessation; the timing, quality, and quantity of rounds required to stop transmission; vaccine stockpile needs; and the impacts of vaccine choices and surveillance quality. We compare the base case scenario that assumes aggressive outbreak response and sufficient mOPV available from the stockpile for all outbreaks that occur in the model, with various scenarios that change the outbreak response strategies.
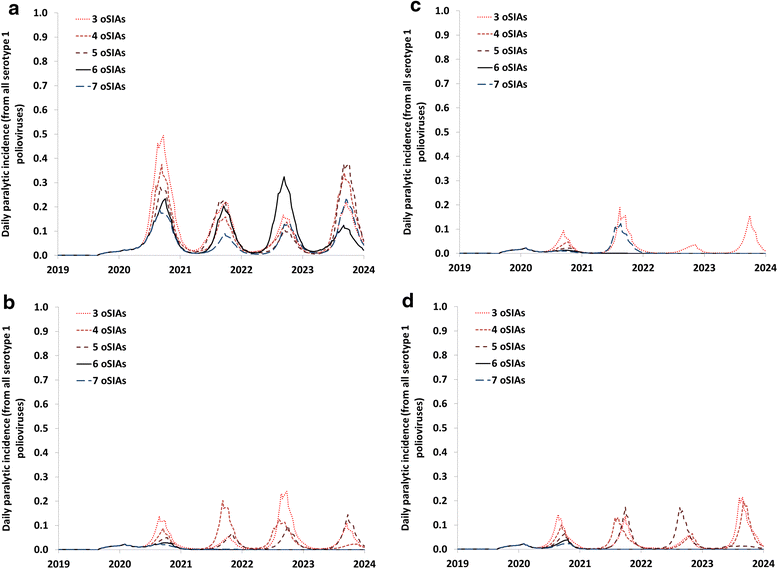
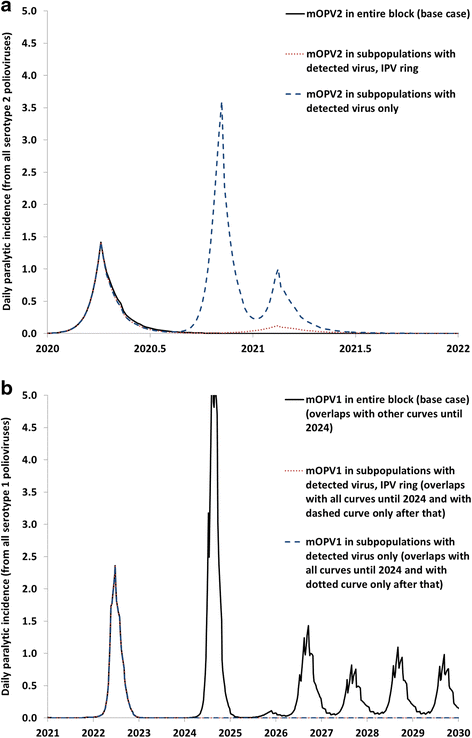
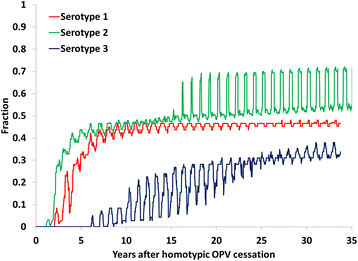
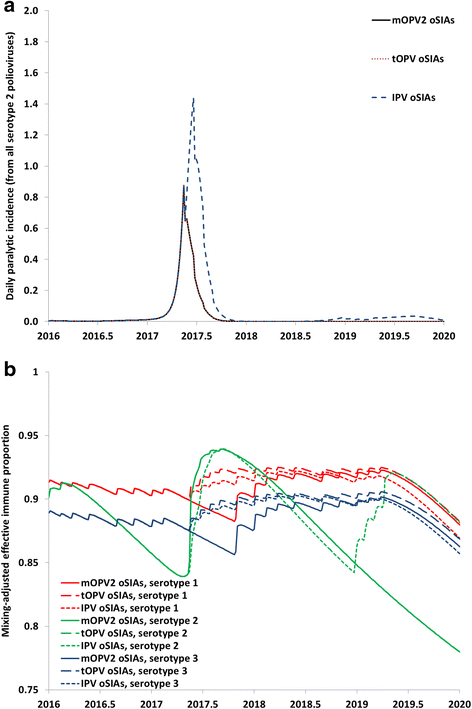
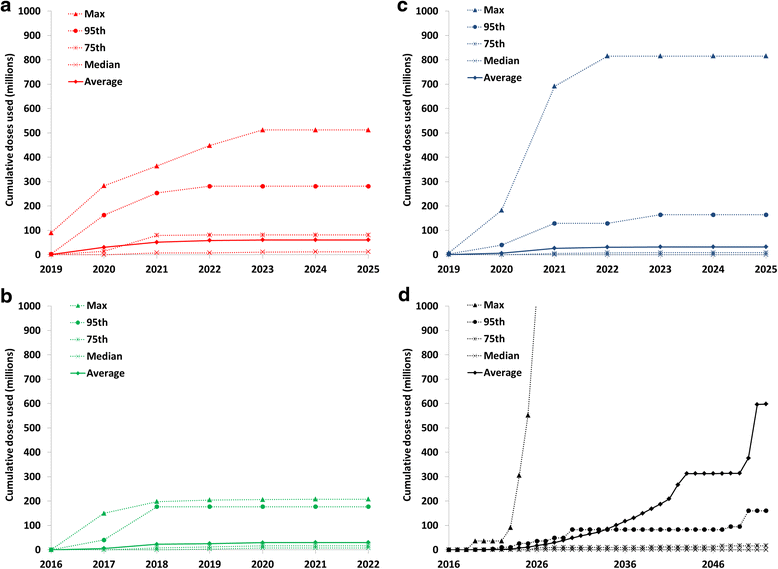
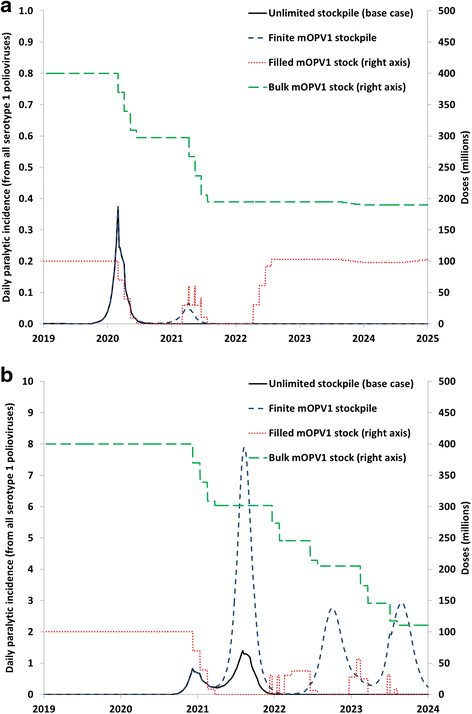
Results: Outbreak response after OPV cessation will require careful management, with some circumstances expected to require more and/or higher quality rounds to stop transmission than others. For outbreaks involving serotype 2, using trivalent OPV instead of mOPV2 following cessation of OPV serotype 2 but before cessation of OPV serotypes 1 and 3 would represent a good option if logistically feasible. Using mOPV for outbreak response can start new outbreaks if exported outside the outbreak population into populations with decreasing population immunity to transmission after OPV cessation, but failure to contain outbreaks resulting in exportation of the outbreak poliovirus may represent a greater risk. The possibility of mOPV use generating new long-term poliovirus excretors represents a real concern. Using the base case outbreak response assumptions, we expect over 25% probability of a shortage of stockpiled filled mOPV vaccine, which could jeopardize the achievement of global polio eradication. For the long term, responding to any poliovirus reintroductions may require a global IPV stockpile. Despite the risks, our model suggests that good risk management and response strategies can successfully control most potential outbreaks after OPV cessation.
Conclusions: Health leaders should carefully consider the numerous outbreak response choices that affect the probability of successfully managing poliovirus risks after OPV cessation.
Keywords: Eradication; Polio; Risk management; Stockpile; Vaccine.
Figures
Similar articles
-
Poliovirus vaccination during the endgame: insights from integrated modeling.Expert Rev Vaccines. 2017 Jun;16(6):577-586. doi: 10.1080/14760584.2017.1322514. Epub 2017 May 9. Expert Rev Vaccines. 2017. PMID: 28437234 Review.
-
The case for cooperation in managing and maintaining the end of poliomyelitis: stockpile needs and coordinated OPV cessation.Medscape J Med. 2008;10(8):190. Epub 2008 Aug 13. Medscape J Med. 2008. PMID: 18924642 Free PMC article.
-
An economic analysis of poliovirus risk management policy options for 2013-2052.BMC Infect Dis. 2015 Sep 24;15:389. doi: 10.1186/s12879-015-1112-8. BMC Infect Dis. 2015. PMID: 26404632 Free PMC article.
-
Health and economic consequences of different options for timing the coordinated global cessation of the three oral poliovirus vaccine serotypes.BMC Infect Dis. 2015 Sep 17;15:374. doi: 10.1186/s12879-015-1113-7. BMC Infect Dis. 2015. PMID: 26381878 Free PMC article.
-
Updated Characterization of Post-OPV Cessation Risks: Lessons from 2019 Serotype 2 Outbreaks and Implications for the Probability of OPV Restart.Risk Anal. 2021 Feb;41(2):320-328. doi: 10.1111/risa.13555. Epub 2020 Jul 6. Risk Anal. 2021. PMID: 32632925 Free PMC article. Review.
Cited by
-
Increasing Population Immunity Prior to Globally-Coordinated Cessation of Bivalent Oral Poliovirus Vaccine (bOPV).Pathogens. 2024 Sep 17;13(9):804. doi: 10.3390/pathogens13090804. Pathogens. 2024. PMID: 39338995 Free PMC article.
-
Review of Poliovirus Transmission and Economic Modeling to Support Global Polio Eradication: 2020-2024.Pathogens. 2024 May 22;13(6):435. doi: 10.3390/pathogens13060435. Pathogens. 2024. PMID: 38921733 Free PMC article. Review.
-
Modeling the spread of circulating vaccine-derived poliovirus type 2 outbreaks and interventions: A case study of Nigeria.Vaccine X. 2024 Mar 16;18:100476. doi: 10.1016/j.jvacx.2024.100476. eCollection 2024 Jun. Vaccine X. 2024. PMID: 38617838 Free PMC article.
-
Modeling Supply and Demand Dynamics of Vaccines against Epidemic-Prone Pathogens: Case Study of Ebola Virus Disease.Vaccines (Basel). 2023 Dec 25;12(1):24. doi: 10.3390/vaccines12010024. Vaccines (Basel). 2023. PMID: 38250837 Free PMC article.
-
Trade-offs of different poliovirus vaccine options for outbreak response in the United States and other countries that only use inactivated poliovirus vaccine (IPV) in routine immunization.Vaccine. 2024 Feb 6;42(4):819-827. doi: 10.1016/j.vaccine.2023.12.081. Epub 2024 Jan 12. Vaccine. 2024. PMID: 38218668 Free PMC article. Review.
References
-
- World Health Organization. Global Polio Eradication Initiative: Polio Eradication and Endgame Strategic Plan (2013–2018). Report number: WHO/POLIO/13.02. Geneva:World Health Organization. 2013.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

