Rab GTPases and the Autophagy Pathway: Bacterial Targets for a Suitable Biogenesis and Trafficking of Their Own Vacuoles
- PMID: 27005665
- PMCID: PMC4810096
- DOI: 10.3390/cells5010011
Rab GTPases and the Autophagy Pathway: Bacterial Targets for a Suitable Biogenesis and Trafficking of Their Own Vacuoles
Abstract
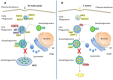
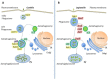
Autophagy is an intracellular process that comprises degradation of damaged organelles, protein aggregates and intracellular pathogens, having an important role in controlling the fate of invading microorganisms. Intracellular pathogens are internalized by professional and non-professional phagocytes, localizing in compartments called phagosomes. To degrade the internalized microorganism, the microbial phagosome matures by fusion events with early and late endosomal compartments and lysosomes, a process that is regulated by Rab GTPases. Interestingly, in order to survive and replicate in the phagosome, some pathogens employ different strategies to manipulate vesicular traffic, inhibiting phagolysosomal biogenesis (e.g., Staphylococcus aureus and Mycobacterium tuberculosis) or surviving in acidic compartments and forming replicative vacuoles (e.g., Coxiella burnetti and Legionella pneumophila). The bacteria described in this review often use secretion systems to control the host's response and thus disseminate. To date, eight types of secretion systems (Type I to Type VIII) are known. Some of these systems are used by bacteria to translocate pathogenic proteins into the host cell and regulate replicative vacuole formation, apoptosis, cytokine responses, and autophagy. Herein, we have focused on how bacteria manipulate small Rab GTPases to control many of these processes. The growing knowledge in this field may facilitate the development of new treatments or contribute to the prevention of these types of bacterial infections.
Keywords: Rab GTPases; autophagy; bacterial pathogens; intracellular bacteria.
Figures
Similar articles
-
Bacteria-Containing Vacuoles: Subversion of Cellular Membrane Traffic and Autophagy.Crit Rev Eukaryot Gene Expr. 2015;25(2):163-74. doi: 10.1615/critreveukaryotgeneexpr.2015013572. Crit Rev Eukaryot Gene Expr. 2015. PMID: 26080610 Review.
-
Taking control: Hijacking of Rab GTPases by intracellular bacterial pathogens.Small GTPases. 2018 Mar 4;9(1-2):182-191. doi: 10.1080/21541248.2017.1336192. Epub 2017 Jul 5. Small GTPases. 2018. PMID: 28632996 Free PMC article. Review.
-
Rab GTPases regulating phagosome maturation are differentially recruited to mycobacterial phagosomes.Traffic. 2011 Apr;12(4):407-20. doi: 10.1111/j.1600-0854.2011.01165.x. Epub 2011 Feb 21. Traffic. 2011. PMID: 21255211
-
Idiosyncratic Biogenesis of Intracellular Pathogens-Containing Vacuoles.Front Cell Infect Microbiol. 2021 Nov 11;11:722433. doi: 10.3389/fcimb.2021.722433. eCollection 2021. Front Cell Infect Microbiol. 2021. PMID: 34858868 Free PMC article. Review.
-
Manipulation of rab GTPase function by intracellular bacterial pathogens.Microbiol Mol Biol Rev. 2007 Dec;71(4):636-52. doi: 10.1128/MMBR.00023-07. Microbiol Mol Biol Rev. 2007. PMID: 18063721 Free PMC article. Review.
Cited by
-
Rab-mediated vesicle trafficking in cancer.J Biomed Sci. 2016 Oct 6;23(1):70. doi: 10.1186/s12929-016-0287-7. J Biomed Sci. 2016. PMID: 27716280 Free PMC article. Review.
-
Analysis of Gtpases Rab 5 and Rab 7 expression from macrophages infected with biofilm-producing and non biofilm-producing strains of Corynebacterium pseudotuberculosis.Braz J Microbiol. 2022 Mar;53(1):447-453. doi: 10.1007/s42770-021-00665-2. Epub 2022 Jan 13. Braz J Microbiol. 2022. PMID: 35023082 Free PMC article.
-
Common Traits Spark the Mitophagy/Xenophagy Interplay.Front Physiol. 2018 Sep 20;9:1172. doi: 10.3389/fphys.2018.01172. eCollection 2018. Front Physiol. 2018. PMID: 30294276 Free PMC article. Review.
-
Fungal mechanisms of intracellular survival: what can we learn from bacterial pathogens?Infect Immun. 2023 Sep 14;91(9):e0043422. doi: 10.1128/iai.00434-22. Epub 2023 Jul 28. Infect Immun. 2023. PMID: 37506189 Free PMC article. Review.
-
Exploring the Role of Staphylococcus aureus in Inflammatory Diseases.Toxins (Basel). 2022 Jul 6;14(7):464. doi: 10.3390/toxins14070464. Toxins (Basel). 2022. PMID: 35878202 Free PMC article. Review.
References
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

