Collagen-binding proteins of Streptococcus mutans and related streptococci
- PMID: 26991416
- PMCID: PMC5025393
- DOI: 10.1111/omi.12158
Collagen-binding proteins of Streptococcus mutans and related streptococci
Abstract
The ability of Streptococcus mutans to interact with collagen through the expression of collagen-binding proteins (CBPs) bestows this oral pathogen with an alternative to the sucrose-dependent mechanism of colonization classically attributed to caries development. Based on the abundance and distribution of collagen throughout the human body, stringent adherence to this molecule grants S. mutans with the opportunity to establish infection at different host sites. Surface proteins, such as SpaP, WapA, Cnm and Cbm, have been shown to bind collagen in vitro, and it has been suggested that these molecules play a role in colonization of oral and extra-oral tissues. However, robust collagen binding is not achieved by all strains of S. mutans, particularly those that lack Cnm or Cbm. These observations merit careful dissection of the contribution from these different CBPs towards tissue colonization and virulence. In this review, we will discuss the current understanding of mechanisms used by S. mutans and related streptococci to colonize collagenous tissues, and the possible contribution of CBPs to infections in different sites of the host.
Keywords: adhesins; collagen binding; streptococcus.
© 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures

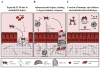
Similar articles
-
CovR and VicRKX Regulate Transcription of the Collagen Binding Protein Cnm of Streptococcus mutans.J Bacteriol. 2018 Nov 6;200(23):e00141-18. doi: 10.1128/JB.00141-18. Print 2018 Dec 1. J Bacteriol. 2018. PMID: 30201780 Free PMC article.
-
Contribution of the Collagen-Binding Proteins of Streptococcus mutans to Bacterial Colonization of Inflamed Dental Pulp.PLoS One. 2016 Jul 21;11(7):e0159613. doi: 10.1371/journal.pone.0159613. eCollection 2016. PLoS One. 2016. PMID: 27442266 Free PMC article.
-
The collagen binding protein Cnm contributes to oral colonization and cariogenicity of Streptococcus mutans OMZ175.Infect Immun. 2015 May;83(5):2001-10. doi: 10.1128/IAI.03022-14. Epub 2015 Mar 2. Infect Immun. 2015. PMID: 25733523 Free PMC article.
-
Current understanding of the cause of dental caries.Jpn J Infect Dis. 2000 Feb;53(1):1-5. Jpn J Infect Dis. 2000. PMID: 10777849 Review.
-
Secretory immunity in defense against cariogenic mutans streptococci.Caries Res. 1999;33(1):4-15. doi: 10.1159/000016490. Caries Res. 1999. PMID: 9831775 Review.
Cited by
-
Clinical characteristics of children and guardians possessing CBP-positive Streptococcus mutans strains: a cross-sectional study.Sci Rep. 2022 Oct 20;12(1):17510. doi: 10.1038/s41598-022-22378-8. Sci Rep. 2022. PMID: 36266432 Free PMC article.
-
The Versatility of Collagen in Pharmacology: Targeting Collagen, Targeting with Collagen.Int J Mol Sci. 2024 Jun 13;25(12):6523. doi: 10.3390/ijms25126523. Int J Mol Sci. 2024. PMID: 38928229 Free PMC article. Review.
-
Post-translational modification by the Pgf glycosylation machinery modulates Streptococcus mutans OMZ175 physiology and virulence.Mol Microbiol. 2024 Aug;122(2):133-151. doi: 10.1111/mmi.15190. Epub 2023 Nov 16. Mol Microbiol. 2024. PMID: 37972006
-
Involvement of the Streptococcus mutans PgfE and GalE 4-epimerases in protein glycosylation, carbon metabolism, and cell division.Glycobiology. 2023 Apr 19;33(3):245-259. doi: 10.1093/glycob/cwad004. Glycobiology. 2023. PMID: 36637425 Free PMC article.
-
Functional Analysis of the Collagen Binding Proteins of Streptococcus parasanguinis FW213.mSphere. 2020 Oct 14;5(5):e00863-20. doi: 10.1128/mSphere.00863-20. mSphere. 2020. PMID: 33055259 Free PMC article.
References
-
- Abercrombie GF, Scott WM. A case of infective endocarditis due to Streptococcus mutans. The Lancet. 1928;212:697–699.
-
- Abu-Qarn M, Eichler J, Sharon N. Not just for Eukarya anymore: protein glycosylation in Bacteria and Archaea. Curr Opin Struct Biol. 2008;18:544–550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

