Initial in vitro studies on tissues and cells from GTKO/CD46/NeuGcKO pigs
- PMID: 26988899
- PMCID: PMC4842123
- DOI: 10.1111/xen.12229
Initial in vitro studies on tissues and cells from GTKO/CD46/NeuGcKO pigs
Abstract
Background: The impact that the absence of expression of NeuGc in pigs might have on pig organ or cell transplantation in humans has been studied in vitro, but only using red blood cells (pRBCs) and peripheral blood mononuclear cells (pPBMCs) as the target cells for immune assays. We have extended this work in various in vitro models and now report our initial results.
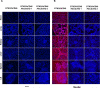
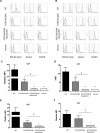
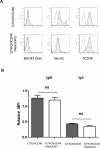
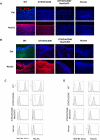
Methods: The models we have used involve GTKO/hCD46 and GTKO/hCD46/NeuGcKO pig aortas and corneas, and pRBCs, pPBMCs, aortic endothelial cells (pAECs), corneal endothelial cells (pCECs), and isolated pancreatic islets. We have investigated the effect of the absence of NeuGc expression on (i) human IgM and IgG binding, (ii) the T-cell proliferative response, (iii) human platelet aggregation, and (iv) in an in vitro assay of the instant blood-mediated inflammatory reaction (IBMIR) following exposure of pig islets to human blood/serum.
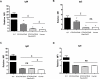
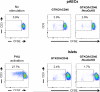
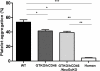
Results: The lack of expression of NeuGc on some pig tissues (aortas, corneas) and cells (RBCs, PBMCs, AECs) significantly reduces the extent of human antibody binding. In contrast, the absence of NeuGc expression on some pig tissues (CECs, isolated islet cells) does not reduce human antibody binding, possibly due to their relatively low NeuGc expression level. The strength of the human T-cell proliferative response may also be marginally reduced, but is already weak to GTKO/hCD46 pAECs and islet cells. We also demonstrate that the absence of NeuGc expression on GTKO/hCD46 pAECs does not reduce human platelet aggregation, and nor does it significantly modify the IBMIR to pig islets.
Conclusion: The absence of NeuGc on some solid organs from GTKO/hCD46/NeuGcKO pigs should reduce the human antibody response after clinical transplantation when compared to GTKO/hCD46 pig organs. However, the clinical benefit of using certain tissue (e.g., cornea, islets) from GTKO/hCD46/NeuGcKO pigs is questionable.
Keywords: Islets; N-glycolylneuraminic acid; Pig; Xenotransplantation; cytidine monophospho-N-acetylneuraminic acid hydroxylase; galactose-α1,3-galactose; pancreatic; α1,3-galactosyltransferase gene-knockout.
© 2016 John Wiley & Sons A/S. Published by John Wiley & Sons Ltd.
Figures
Similar articles
-
Expression of NeuGc on Pig Corneas and Its Potential Significance in Pig Corneal Xenotransplantation.Cornea. 2016 Jan;35(1):105-13. doi: 10.1097/ICO.0000000000000635. Cornea. 2016. PMID: 26418433 Free PMC article.
-
Human antibody recognition of xenogeneic antigens (NeuGc and Gal) on porcine heart valves: could genetically modified pig heart valves reduce structural valve deterioration?Xenotransplantation. 2016 Sep;23(5):370-80. doi: 10.1111/xen.12254. Epub 2016 Aug 11. Xenotransplantation. 2016. PMID: 27511593
-
Initial in vitro investigation of the human immune response to corneal cells from genetically engineered pigs.Invest Ophthalmol Vis Sci. 2011 Jul 15;52(8):5278-86. doi: 10.1167/iovs.10-6947. Invest Ophthalmol Vis Sci. 2011. PMID: 21596821 Free PMC article.
-
Modifying the sugar icing on the transplantation cake.Glycobiology. 2016 Jun;26(6):571-81. doi: 10.1093/glycob/cww028. Epub 2016 Mar 1. Glycobiology. 2016. PMID: 26935763 Free PMC article. Review.
-
The pathobiology of pig-to-primate xenotransplantation: a historical review.Xenotransplantation. 2016 Mar;23(2):83-105. doi: 10.1111/xen.12219. Epub 2016 Jan 27. Xenotransplantation. 2016. PMID: 26813438 Review.
Cited by
-
Progress in Clinical Encapsulated Islet Xenotransplantation.Transplantation. 2016 Nov;100(11):2301-2308. doi: 10.1097/TP.0000000000001371. Transplantation. 2016. PMID: 27482959 Free PMC article. Review.
-
Perspectives on the Optimal Genetically Engineered Pig in 2018 for Initial Clinical Trials of Kidney or Heart Xenotransplantation.Transplantation. 2018 Dec;102(12):1974-1982. doi: 10.1097/TP.0000000000002443. Transplantation. 2018. PMID: 30247446 Free PMC article. Review.
-
Effect of intravenous immunoglobulin (IVIg) on primate complement-dependent cytotoxicity of genetically engineered pig cells: relevance to clinical xenotransplantation.Sci Rep. 2020 Jul 16;10(1):11747. doi: 10.1038/s41598-020-68505-1. Sci Rep. 2020. PMID: 32678137 Free PMC article.
-
N-glycolylneuraminic acid knockout reduces erythrocyte sequestration and thromboxane elaboration in an ex vivo pig-to-human xenoperfusion model.Xenotransplantation. 2017 Nov;24(6):10.1111/xen.12339. doi: 10.1111/xen.12339. Epub 2017 Sep 22. Xenotransplantation. 2017. PMID: 28940313 Free PMC article.
-
Current Topics of Relevance to the Xenotransplantation of Free Pig Islets.Front Immunol. 2022 Apr 1;13:854883. doi: 10.3389/fimmu.2022.854883. eCollection 2022. Front Immunol. 2022. PMID: 35432379 Free PMC article. Review.
References
-
- GOOD AH, COOPER DK, MALCOLM AJ, IPPOLITO RM, KOREN E, NEETHLING FA, et al. Identification of carbohydrate structures that bind human antiporcine antibodies: implications for discordant xenografting in humans. Transplant Proc. 1992;24:559–562. - PubMed
-
- COOPER DK, GOOD AH, KOREN E, ORIOL R, MALCOLM AJ, IPPOLITO RM, et al. Identification of alpha-galactosyl and other carbohydrate epitopes that are bound by human anti-pig antibodies: relevance to discordant xenografting in man. Transpl Immunol. 1993;1:198–205. - PubMed
-
- BOUHOURS D, POURCEL C, BOUHOURS J-F. Simultaneous expression by porcine aorta endothelial cells of glycosphingolipids bearing the major epitope for human xenoreactive antibodies (Galα1–3Gal), blood group H determinant andN-glycolylneuraminic acid. Glycoconj J. 1996;13:947–953. - PubMed
-
- ZHU A, HURST R. Anti-N-glycolylneuraminic acid antibodies identified in healthy human serum. Xenotransplantation. 2002;9:376–381. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

