Doxorubicin Blocks Cardiomyocyte Autophagic Flux by Inhibiting Lysosome Acidification
- PMID: 26984939
- PMCID: PMC4856587
- DOI: 10.1161/CIRCULATIONAHA.115.017443
Doxorubicin Blocks Cardiomyocyte Autophagic Flux by Inhibiting Lysosome Acidification
Abstract
Background: The clinical use of doxorubicin is limited by cardiotoxicity. Histopathological changes include interstitial myocardial fibrosis and the appearance of vacuolated cardiomyocytes. Whereas dysregulation of autophagy in the myocardium has been implicated in a variety of cardiovascular diseases, the role of autophagy in doxorubicin cardiomyopathy remains poorly defined.
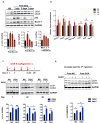
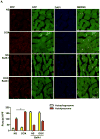
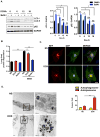
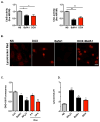
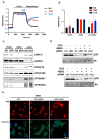
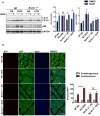
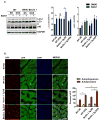
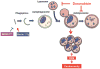
Methods and results: Most models of doxorubicin cardiotoxicity involve intraperitoneal injection of high-dose drug, which elicits lethargy, anorexia, weight loss, and peritoneal fibrosis, all of which confound the interpretation of autophagy. Given this, we first established a model that provokes modest and progressive cardiotoxicity without constitutional symptoms, reminiscent of the effects seen in patients. We report that doxorubicin blocks cardiomyocyte autophagic flux in vivo and in cardiomyocytes in culture. This block was accompanied by robust accumulation of undegraded autolysosomes. We go on to localize the site of block as a defect in lysosome acidification. To test the functional relevance of doxorubicin-triggered autolysosome accumulation, we studied animals with diminished autophagic activity resulting from haploinsufficiency for Beclin 1. Beclin 1(+/-) mice exposed to doxorubicin were protected in terms of structural and functional changes within the myocardium. Conversely, animals overexpressing Beclin 1 manifested an amplified cardiotoxic response.
Conclusions: Doxorubicin blocks autophagic flux in cardiomyocytes by impairing lysosome acidification and lysosomal function. Reducing autophagy initiation protects against doxorubicin cardiotoxicity.
Keywords: autophagy; cardiotoxicity; doxorubicin; drug therapy; myocytes, cardiac.
© 2016 American Heart Association, Inc.
Figures


Similar articles
-
Nicotinamide riboside promotes autolysosome clearance in preventing doxorubicin-induced cardiotoxicity.Clin Sci (Lond). 2019 Jul 15;133(13):1505-1521. doi: 10.1042/CS20181022. Print 2019 Jul 15. Clin Sci (Lond). 2019. PMID: 31266854 Free PMC article.
-
Doxorubicin impairs cardiomyocyte viability by suppressing transcription factor EB expression and disrupting autophagy.Biochem J. 2016 Nov 1;473(21):3769-3789. doi: 10.1042/BCJ20160385. Epub 2016 Aug 3. Biochem J. 2016. PMID: 27487838
-
Autophagic dysregulation in doxorubicin cardiomyopathy.J Mol Cell Cardiol. 2017 Mar;104:1-8. doi: 10.1016/j.yjmcc.2017.01.007. Epub 2017 Jan 17. J Mol Cell Cardiol. 2017. PMID: 28108310 Review.
-
Evidences for the mechanism of Shenmai injection antagonizing doxorubicin-induced cardiotoxicity.Phytomedicine. 2021 Jul 15;88:153597. doi: 10.1016/j.phymed.2021.153597. Epub 2021 May 21. Phytomedicine. 2021. PMID: 34111614
-
Autophagy and mitophagy in the context of doxorubicin-induced cardiotoxicity.Oncotarget. 2017 Jul 11;8(28):46663-46680. doi: 10.18632/oncotarget.16944. Oncotarget. 2017. PMID: 28445146 Free PMC article. Review.
Cited by
-
Thymoquinone‑induced autophagy mitigates doxorubicin‑induced H9c2 cell apoptosis.Exp Ther Med. 2022 Sep 28;24(5):694. doi: 10.3892/etm.2022.11630. eCollection 2022 Nov. Exp Ther Med. 2022. PMID: 36277157 Free PMC article.
-
Potential role of endoplasmic reticulum stress in doxorubicin-induced cardiotoxicity-an update.Front Pharmacol. 2024 Aug 12;15:1415108. doi: 10.3389/fphar.2024.1415108. eCollection 2024. Front Pharmacol. 2024. PMID: 39188945 Free PMC article. Review.
-
Regulated cell death pathways in cardiomyopathy.Acta Pharmacol Sin. 2023 Aug;44(8):1521-1535. doi: 10.1038/s41401-023-01068-9. Epub 2023 Mar 13. Acta Pharmacol Sin. 2023. PMID: 36914852 Free PMC article. Review.
-
The role of heat shock proteins in the pathogenesis of heart failure (Review).Int J Mol Med. 2023 Nov;52(5):106. doi: 10.3892/ijmm.2023.5309. Epub 2023 Sep 29. Int J Mol Med. 2023. PMID: 37772383 Free PMC article. Review.
-
Inositol pyrophosphates mediated the apoptosis induced by hypoxic injury in bone marrow-derived mesenchymal stem cells by autophagy.Stem Cell Res Ther. 2019 Jun 3;10(1):159. doi: 10.1186/s13287-019-1256-3. Stem Cell Res Ther. 2019. PMID: 31159888 Free PMC article.
References
-
- Singal PK, Deally CM, Weinberg LE. Subcellular effects of adriamycin in the heart: A concise review. J Mol Cell Cardiol. 1987;19:817–828. - PubMed
-
- Lipshultz SE, Colan SD, Gelber RD, Perez-Atayde AR, Sallan SE, Sanders SP. Late cardiac effects of doxorubicin therapy for acute lymphoblastic leukemia in childhood. N Engl J Med. 1991;324:808–815. - PubMed
-
- Octavia Y, Tocchetti CG, Gabrielson KL, Janssens S, Crijns HJ, Moens AL. Doxorubicin-induced cardiomyopathy: From molecular mechanisms to therapeutic strategies. J Mol Cell Cardiol. 2012;52:1213–1225. - PubMed
-
- Zhang S, Liu X, Bawa-Khalfe T, Lu LS, Lyu YL, Liu LF, Yeh ET. Identification of the molecular basis of doxorubicin-induced cardiotoxicity. Nat Med. 2012;18:1639–1642. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

