Mass spectrometric analysis of accumulated TDP-43 in amyotrophic lateral sclerosis brains
- PMID: 26980269
- PMCID: PMC4793195
- DOI: 10.1038/srep23281
Mass spectrometric analysis of accumulated TDP-43 in amyotrophic lateral sclerosis brains
Abstract
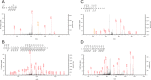
TDP-43 is the major disease-associated protein involved in the pathogenesis and progression of amyotrophic lateral sclerosis (ALS) and frontotemporal lobar degeneration with ubiquitin-positive inclusions linked to TDP-43 pathology (FTLD-TDP). Abnormal phosphorylation, truncation and cytoplasmic mis-localization are known to be the characteristics for the aggregated forms of TDP-43, and gain of toxic abnormal TDP-43 or loss of function of physiological TDP-43 have been suggested as the cause of neurodegeneration. However, most of the post-translational modifications or truncation sites in the abnormal TDP-43 in brains of patients remain to be identified by protein chemical analysis. In this study, we carried out a highly sensitive liquid chromatography-mass spectrometry analysis of Sarkosyl-insoluble pathological TDP-43 from brains of ALS patients and identified several novel phosphorylation sites, deamidation sites, and cleavage sites. Almost all modifications were localized in the Gly-rich C-terminal half. Most of the cleavage sites identified in this study are novel and are located in N-terminal half, suggesting that these sites may be more accessible to proteolytic enzymes. The data obtained in this study provide a foundation for the molecular mechanisms of TDP-43 aggregation and ALS pathogenesis.
Figures



Similar articles
-
Phosphorylated TDP-43 in frontotemporal lobar degeneration and amyotrophic lateral sclerosis.Ann Neurol. 2008 Jul;64(1):60-70. doi: 10.1002/ana.21425. Ann Neurol. 2008. PMID: 18546284 Free PMC article.
-
TDP-43 is a component of ubiquitin-positive tau-negative inclusions in frontotemporal lobar degeneration and amyotrophic lateral sclerosis.Biochem Biophys Res Commun. 2006 Dec 22;351(3):602-11. doi: 10.1016/j.bbrc.2006.10.093. Epub 2006 Oct 30. Biochem Biophys Res Commun. 2006. PMID: 17084815
-
Abnormal phosphorylation of Ser409/410 of TDP-43 in FTLD-U and ALS.FEBS Lett. 2008 Aug 20;582(19):2899-904. doi: 10.1016/j.febslet.2008.07.027. Epub 2008 Jul 24. FEBS Lett. 2008. PMID: 18656473
-
Phosphorylated and cleaved TDP-43 in ALS, FTLD and other neurodegenerative disorders and in cellular models of TDP-43 proteinopathy.Neuropathology. 2010 Apr;30(2):170-81. doi: 10.1111/j.1440-1789.2009.01089.x. Epub 2010 Jan 19. Neuropathology. 2010. PMID: 20102522 Review.
-
Possible concurrence of TDP-43, tau and other proteins in amyotrophic lateral sclerosis/frontotemporal lobar degeneration.Neuropathology. 2018 Feb;38(1):72-81. doi: 10.1111/neup.12428. Epub 2017 Sep 27. Neuropathology. 2018. PMID: 28960544 Review.
Cited by
-
Regulation of TDP-43 phosphorylation in aging and disease.Geroscience. 2021 Aug;43(4):1605-1614. doi: 10.1007/s11357-021-00383-5. Epub 2021 May 25. Geroscience. 2021. PMID: 34032984 Free PMC article.
-
TDP-35, a truncated fragment of TDP-43, induces dose-dependent toxicity and apoptosis in flies.Neural Regen Res. 2022 Nov;17(11):2441-2442. doi: 10.4103/1673-5374.338997. Neural Regen Res. 2022. PMID: 35535891 Free PMC article. No abstract available.
-
TDP-43 and HERV-K Envelope-Specific Immunogenic Epitopes Are Recognized in ALS Patients.Viruses. 2021 Nov 18;13(11):2301. doi: 10.3390/v13112301. Viruses. 2021. PMID: 34835107 Free PMC article.
-
Tandem detergent-extraction and immunoprecipitation of proteinopathy: Scalable enrichment of ALS-associated TDP-43 aggregates.iScience. 2023 Apr 11;26(5):106645. doi: 10.1016/j.isci.2023.106645. eCollection 2023 May 19. iScience. 2023. PMID: 37182104 Free PMC article.
-
N-Terminal Fragments of TDP-43-In Vitro Analysis and Implication in the Pathophysiology of Amyotrophic Lateral Sclerosis and Frontotemporal Lobar Degeneration.Genes (Basel). 2024 Sep 1;15(9):1157. doi: 10.3390/genes15091157. Genes (Basel). 2024. PMID: 39336748 Free PMC article.
References
-
- Corrado L. et al. High frequency of TARDBP gene mutations in Italian patients with amyotrophic lateral sclerosis. Human Mutation 30, 688–694 (2009). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

