Secretory leukocyte protease inhibitor gene deletion alters bleomycin-induced lung injury, but not development of pulmonary fibrosis
- PMID: 26974397
- PMCID: PMC4884449
- DOI: 10.1038/labinvest.2016.40
Secretory leukocyte protease inhibitor gene deletion alters bleomycin-induced lung injury, but not development of pulmonary fibrosis
Abstract
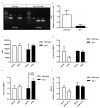
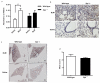
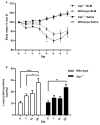
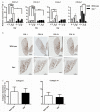
Idiopathic pulmonary fibrosis is a progressive, fatal disease with limited treatment options. Protease-mediated transforming growth factor-β (TGF-β) activation has been proposed as a pathogenic mechanism of lung fibrosis. Protease activity in the lung is tightly regulated by protease inhibitors, particularly secretory leukocyte protease inhibitor (SLPI). The bleomycin model of lung fibrosis was used to determine the effect of increased protease activity in the lungs of Slpi(-/-) mice following injury. Slpi(-/-), and wild-type, mice received oropharyngeal administration of bleomycin (30 IU) and the development of pulmonary fibrosis was assessed. Pro and active forms of matrix metalloproteinase (MMP)-2 and MMP-9 were measured. Lung fibrosis was determined by collagen subtype-specific gene expression, hydroxyproline concentration, and histological assessment. Alveolar TGF-β activation was measured using bronchoalveolar lavage cell pSmad2 levels and global TGF-β activity was assessed by pSmad2 immunohistochemistry. The active-MMP-9 to pro-MMP-9 ratio was significantly increased in Slpi(-/-) animals compared with wild-type animals, demonstrating enhanced metalloproteinase activity. Wild-type animals showed an increase in TGF-β activation following bleomycin, with a progressive and sustained increase in collagen type I, alpha 1 (Col1α1), III, alpha 1(Col3α1), IV, alpha 1(Col4α1) mRNA expression, and a significant increase in total lung collagen 28 days post bleomycin. In contrast Slpi(-/-) mice showed no significant increase of alveolar TGF-β activity following bleomycin, above their already elevated levels, although global TGF-β activity did increase. Slpi(-/-) mice had impaired collagen gene expression but animals demonstrated minimal reduction in lung fibrosis compared with wild-type animals. These data suggest that enhanced proteolysis does not further enhance TGF-β activation, and inhibits sustained Col1α1, Col3α1, and Col4α1 gene expression following lung injury. However, these changes do not prevent the development of lung fibrosis. Overall, these data suggest that the absence of Slpi does not markedly modify the development of lung fibrosis following bleomycin-induced lung injury.
Figures
Similar articles
-
Lysophosphatidic acid receptor-2 deficiency confers protection against bleomycin-induced lung injury and fibrosis in mice.Am J Respir Cell Mol Biol. 2013 Dec;49(6):912-22. doi: 10.1165/rcmb.2013-0070OC. Am J Respir Cell Mol Biol. 2013. PMID: 23808384 Free PMC article.
-
Amelioration of bleomycin-induced pulmonary fibrosis in a mouse model by a combination therapy of bosentan and imatinib.Exp Lung Res. 2015 May;41(4):173-88. doi: 10.3109/01902148.2014.939312. Epub 2015 Apr 6. Exp Lung Res. 2015. PMID: 25844688
-
Tubastatin ameliorates pulmonary fibrosis by targeting the TGFβ-PI3K-Akt pathway.PLoS One. 2017 Oct 18;12(10):e0186615. doi: 10.1371/journal.pone.0186615. eCollection 2017. PLoS One. 2017. PMID: 29045477 Free PMC article.
-
Pulmonary fibrosis and type-17 immunity.Respir Investig. 2023 Sep;61(5):553-562. doi: 10.1016/j.resinv.2023.05.005. Epub 2023 Jun 23. Respir Investig. 2023. PMID: 37356133 Review.
-
Secretory leukocyte peptidase inhibitor and lung cancer.Cancer Sci. 2008 May;99(5):849-55. doi: 10.1111/j.1349-7006.2008.00772.x. Cancer Sci. 2008. PMID: 18380788 Free PMC article. Review.
Cited by
-
Host-Microbial Interactions in Idiopathic Pulmonary Fibrosis.Am J Respir Crit Care Med. 2017 Jun 15;195(12):1640-1650. doi: 10.1164/rccm.201607-1408OC. Am J Respir Crit Care Med. 2017. PMID: 28085486 Free PMC article.
-
Matrix Metalloproteinases and Their Inhibitors in Pulmonary Fibrosis: EMMPRIN/CD147 Comes into Play.Int J Mol Sci. 2022 Jun 21;23(13):6894. doi: 10.3390/ijms23136894. Int J Mol Sci. 2022. PMID: 35805895 Free PMC article. Review.
-
Gene-based evaluation of low-frequency variation and genetically-predicted gene expression impacting risk of keloid formation.Ann Hum Genet. 2018 Jul;82(4):206-215. doi: 10.1111/ahg.12245. Epub 2018 Feb 27. Ann Hum Genet. 2018. PMID: 29484647 Free PMC article.
-
Pathobiology of Airway Remodeling in Asthma: The Emerging Role of Integrins.J Asthma Allergy. 2022 May 11;15:595-610. doi: 10.2147/JAA.S267222. eCollection 2022. J Asthma Allergy. 2022. PMID: 35592385 Free PMC article. Review.
-
Reviewing the Regulators of COL1A1.Int J Mol Sci. 2023 Jun 11;24(12):10004. doi: 10.3390/ijms241210004. Int J Mol Sci. 2023. PMID: 37373151 Free PMC article. Review.
References
-
- Maher TM, Wells AU, Laurent GJ. Idiopathic pulmonary fibrosis: multiple causes and multiple mechanisms? Eur Respir J. 2007;30(5):835–839. - PubMed
-
- Selman M, King TE, Pardo A. Idiopathic pulmonary fibrosis: prevailing and evolving hypotheses about its pathogenesis and implications for therapy. Ann Intern Med. 2001;134(2):136–151. - PubMed
-
- Kinder BW, Brown KK, Schwarz MI, et al. Baseline BAL neutrophilia predicts early mortality in idiopathic pulmonary fibrosis. Chest. 2008;133(1):226–232. - PubMed
-
- Pham CT. Neutrophil serine proteases: specific regulators of inflammation. Nat Rev Immunol. 2006;6(7):541–550. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

