Acetylation and deacetylation of Cdc25A constitutes a novel mechanism for modulating Cdc25A functions with implications for cancer
- PMID: 26967250
- PMCID: PMC4991465
- DOI: 10.18632/oncotarget.7966
Acetylation and deacetylation of Cdc25A constitutes a novel mechanism for modulating Cdc25A functions with implications for cancer
Abstract
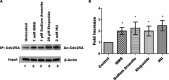
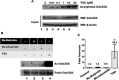
The dual specificity phosphatase Cdc25A is a key regulator of the cell cycle that promotes cell cycle progression by dephosphorylating and activating cyclin-dependent kinases. In response to genotoxicants, Cdc25A undergoes posttranslational modifications which contribute to its proteasome-mediated degradation and consequent cell cycle checkpoint arrest. The most thoroughly studied Cdc25A modification is phosphorylation. We now provide the first evidence that Cdc25A can be acetylated and that it directly interacts with the ARD1 acetyltransferase which acetylates Cdc25A both biochemically and in cultured cells. When acetylated, Cdc25A has an extended half-life. We have also identified the class IV histone deacetylase, HDAC11, as a Cdc25A deacetylase. We further show that DNA damage, such as exposure to methyl methanesulfonate (MMS), etoposide or arsenic, increases Cdc25A acetylation. Importantly, this acetylation modulates Cdc25A phosphatase activity and its function as a cell cycle regulator, and may reflect a cellular response to DNA damage. Since Cdc25A, ARD1, and HDAC11 are frequently dysregulated in multiple types of cancer, our findings may provide insight into a novel mechanism in carcinogenesis.
Keywords: ARD1; Cdc25A acetylation; DNA damage; HDAC11; cancer.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

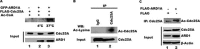



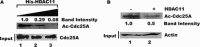
Similar articles
-
Diverse roles of arrest defective 1 in cancer development.Arch Pharm Res. 2019 Dec;42(12):1040-1051. doi: 10.1007/s12272-019-01195-0. Epub 2019 Dec 7. Arch Pharm Res. 2019. PMID: 31813105 Review.
-
Versatility of ARD1/NAA10-mediated protein lysine acetylation.Exp Mol Med. 2018 Jul 27;50(7):1-13. doi: 10.1038/s12276-018-0100-7. Exp Mol Med. 2018. PMID: 30054464 Free PMC article. Review.
-
Ubiquitin hydrolase Dub3 promotes oncogenic transformation by stabilizing Cdc25A.Nat Cell Biol. 2010 Apr;12(4):400-6. doi: 10.1038/ncb2041. Epub 2010 Mar 14. Nat Cell Biol. 2010. PMID: 20228808
-
Serine-Threonine Kinase 38 regulates CDC25A stability and the DNA damage-induced G2/M checkpoint.Cell Signal. 2015 Aug;27(8):1569-75. doi: 10.1016/j.cellsig.2015.04.013. Epub 2015 Apr 30. Cell Signal. 2015. PMID: 25936524
-
ARD1 contributes to IKKβ-mediated breast cancer tumorigenesis.Cell Death Dis. 2018 Aug 28;9(9):860. doi: 10.1038/s41419-018-0921-2. Cell Death Dis. 2018. PMID: 30154412 Free PMC article.
Cited by
-
Co-translational, Post-translational, and Non-catalytic Roles of N-Terminal Acetyltransferases.Mol Cell. 2019 Mar 21;73(6):1097-1114. doi: 10.1016/j.molcel.2019.02.007. Epub 2019 Mar 13. Mol Cell. 2019. PMID: 30878283 Free PMC article. Review.
-
NAA10 as a New Prognostic Marker for Cancer Progression.Int J Mol Sci. 2020 Oct 28;21(21):8010. doi: 10.3390/ijms21218010. Int J Mol Sci. 2020. PMID: 33126484 Free PMC article. Review.
-
Post-Translational Modifications by Lipid Metabolites during the DNA Damage Response and Their Role in Cancer.Biomolecules. 2022 Nov 8;12(11):1655. doi: 10.3390/biom12111655. Biomolecules. 2022. PMID: 36359005 Free PMC article. Review.
-
[Overexpression of histone deacetylase 11 suppresses basal-like breast cancer cell invasion and metastasis].Nan Fang Yi Ke Da Xue Xue Bao. 2019 Jul 30;39(7):751-759. doi: 10.12122/j.issn.1673-4254.2019.07.01. Nan Fang Yi Ke Da Xue Xue Bao. 2019. PMID: 31340905 Free PMC article.
-
ARD1/NAA10 acetylation in prostate cancer.Exp Mol Med. 2018 Jul 27;50(7):1-8. doi: 10.1038/s12276-018-0107-0. Exp Mol Med. 2018. PMID: 30054487 Free PMC article. Review.
References
-
- Ray D, Terao Y, Nimbalkar D, Hirai H, Osmundson EC, Zou X, Franks R, Christov K, Kiyokawa H. Hemizygous disruption of Cdc25A inhibits cellular transformation and mammary tumorigenesis in mice. Cancer Res. 2007;67:6605–11. - PubMed
-
- Lincoln AJ, Wickramasinghe D, Stein P, Schultz RM, Palko ME, De Miguel MP, Tessarollo L, Donovan PJ. Cdc25b phosphatase is required for resumption of meiosis during oocyte maturation. Nat Genet. 2002;30:446–9. - PubMed
-
- Sengupta S, Jana S, Bhattacharyya A. TGF-β-Smad2 dependent activation of CDC 25A plays an important role in cell proliferation through NFAT activation in metastatic breast cancer cells. Cell Signal. 2014;26:240–52. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

