SNX9 promotes metastasis by enhancing cancer cell invasion via differential regulation of RhoGTPases
- PMID: 26960793
- PMCID: PMC4850029
- DOI: 10.1091/mbc.E16-02-0101
SNX9 promotes metastasis by enhancing cancer cell invasion via differential regulation of RhoGTPases
Abstract
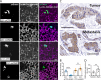
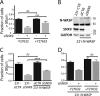
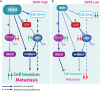
Despite current advances in cancer research, metastasis remains the leading factor in cancer-related deaths. Here, we identify sorting nexin 9 (SNX9) as a new regulator of breast cancer metastasis. We detected an increase in SNX9 expression in human breast cancer metastases compared with primary tumors and demonstrated that SNX9 expression in MDA-MB-231 breast cancer cells is necessary to maintain their ability to metastasize in a chick embryo model. Reciprocally, SNX9 knockdown impairs the process. In vitro studies using several cancer cell lines derived from a variety of human tumors revealed a role for SNX9 in cell invasion and identified mechanisms responsible for this novel function. We showed that SNX9 controls the activation of RhoA and Cdc42 GTPases and also regulates cell motility via the modulation of well-known molecules involved in metastasis, namely RhoA-ROCK and N-WASP. In addition, we have discovered that SNX9 is required for RhoGTPase-dependent, clathrin-independent endocytosis, and in this capacity, can functionally substitute to the bona fide Rho GAP, GRAF1 (GTPase Regulator Associated with Focal Adhesion Kinase). Together, our data establish novel roles for SNX9 as a multifunctional protein scaffold that regulates, and potentially coordinates, several cellular processes that together can enhance cancer cell metastasis.
© 2016 by The American Society for Cell Biology.
Figures
Similar articles
-
Endocytosis, Metastasis and Beyond: Multiple Facets of SNX9.Trends Cell Biol. 2017 Mar;27(3):189-200. doi: 10.1016/j.tcb.2016.11.001. Epub 2016 Dec 16. Trends Cell Biol. 2017. PMID: 27989654 Free PMC article. Review.
-
Sorting nexin 9 negatively regulates invadopodia formation and function in cancer cells.J Cell Sci. 2016 Jul 15;129(14):2804-16. doi: 10.1242/jcs.188045. Epub 2016 Jun 8. J Cell Sci. 2016. PMID: 27278018 Free PMC article.
-
Sorting nexin 9 interacts with dynamin 1 and N-WASP and coordinates synaptic vesicle endocytosis.J Biol Chem. 2007 Sep 28;282(39):28939-28950. doi: 10.1074/jbc.M700283200. Epub 2007 Aug 6. J Biol Chem. 2007. PMID: 17681954
-
Interaction of the Wiskott-Aldrich syndrome protein with sorting nexin 9 is required for CD28 endocytosis and cosignaling in T cells.Proc Natl Acad Sci U S A. 2007 Jan 30;104(5):1593-8. doi: 10.1073/pnas.0610543104. Epub 2007 Jan 22. Proc Natl Acad Sci U S A. 2007. PMID: 17242350 Free PMC article.
-
SNX9 - a prelude to vesicle release.J Cell Sci. 2009 Jan 1;122(Pt 1):5-11. doi: 10.1242/jcs.037135. J Cell Sci. 2009. PMID: 19092055 Review.
Cited by
-
An image-based assay to quantify changes in proliferation and viability upon drug treatment in 3D microenvironments.BMC Cancer. 2019 May 28;19(1):502. doi: 10.1186/s12885-019-5694-1. BMC Cancer. 2019. PMID: 31138163 Free PMC article.
-
SNX20 Expression Correlates with Immune Cell Infiltration and Can Predict Prognosis in Lung Adenocarcinoma.Int J Gen Med. 2021 Nov 3;14:7599-7611. doi: 10.2147/IJGM.S337198. eCollection 2021. Int J Gen Med. 2021. PMID: 34764676 Free PMC article.
-
Identification of a Human Airway Epithelial Cell Subpopulation with Altered Biophysical, Molecular, and Metastatic Properties.Cancer Prev Res (Phila). 2017 Sep;10(9):514-524. doi: 10.1158/1940-6207.CAPR-16-0335. Epub 2017 Jul 28. Cancer Prev Res (Phila). 2017. PMID: 28754664 Free PMC article.
-
Nck deficiency is associated with delayed breast carcinoma progression and reduced metastasis.Mol Biol Cell. 2017 Nov 15;28(24):3500-3516. doi: 10.1091/mbc.E17-02-0106. Epub 2017 Sep 27. Mol Biol Cell. 2017. PMID: 28954862 Free PMC article.
-
A noncanonical role for dynamin-1 in regulating early stages of clathrin-mediated endocytosis in non-neuronal cells.PLoS Biol. 2018 Apr 18;16(4):e2005377. doi: 10.1371/journal.pbio.2005377. eCollection 2018 Apr. PLoS Biol. 2018. PMID: 29668686 Free PMC article.
References
-
- Bacac M, Stamenkovic I. Metastatic cancer cell. Annu Rev Pathol. 2008;3:221–247. - PubMed
-
- Bellizzi A, Mangia A, Chiriatti A, Petroni S, Quaranta M, Schittulli F, Malfettone A, Cardone RA, Paradiso A, Reshkin SJ. RhoA protein expression in primary breast cancers and matched lymphocytes is associated with progression of the disease. Int J Mol Med. 2008;22:25–31. - PubMed
-
- Borm B, Requardt RP, Herzog V, Kirfel G. Membrane ruffles in cell migration: indicators of inefficient lamellipodia adhesion and compartments of actin filament reorganization. Exp Cell Res. 2005;302:83–95. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

