Smoking-promoted oxidative DNA damage response is highly correlated to lung carcinogenesis
- PMID: 26942876
- PMCID: PMC4951340
- DOI: 10.18632/oncotarget.7810
Smoking-promoted oxidative DNA damage response is highly correlated to lung carcinogenesis
Abstract
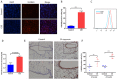
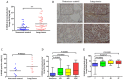
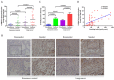
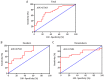
Oxidative stress induced by tobacco smoking is one of the main causes of DNA damage and is known to be involved in various cancers. Smoking is the leading cause of lung cancer, while the role of cigarette smoke-induced oxidative DNA damage response during lung carcinogenesis is largely unknown. In this study, we investigated oxidative DNA damage response levels in smoking and nonsmoking patients with lung cancer, and evaluated the potential diagnostic value of 8-OHdG for lung cancer. We observed a higher level of 8-OHdG expression and secretion in airways of lung cancer patients than that of noncancer controls. 8-OHdG expression was associated with the TNM stages. Additionally, cigarette smoke-induced oxidative DNA damage response was observed in bronchial epithelial cells in vitro and in vivo. A statistical significance correlation was found between the levels of 8-OHdG and smoking index. With a cut-off value of 2.86 ng/ml, 8-OHdG showed a sensitivity and specificity of 70.0% and 73.7%, respectively, to identify a patient with lung cancer. These findings not only underscore the importance of smoking in oxidative DNA damage response of lung cancer patients, but also suggest 8-OHdG as a potential diagnostic biomarker for lung cancer.
Keywords: 8-OHdG; biomarker; lung cancer; oxidative DNA damage; smoking.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
Similar articles
-
Oxidative DNA damage is involved in cigarette smoke-induced lung injury in rats.Environ Health Prev Med. 2015 Sep;20(5):318-24. doi: 10.1007/s12199-015-0469-z. Epub 2015 May 13. Environ Health Prev Med. 2015. PMID: 25967734 Free PMC article.
-
Oxidative DNA damage in human esophageal cancer: clinicopathological analysis of 8-hydroxydeoxyguanosine and its repair enzyme.Dis Esophagus. 2014 Apr;27(3):285-93. doi: 10.1111/dote.12107. Epub 2013 Aug 1. Dis Esophagus. 2014. PMID: 23902537
-
Leanness, smoking, and enhanced oxidative DNA damage.Cancer Epidemiol Biomarkers Prev. 2006 Mar;15(3):582-5. doi: 10.1158/1055-9965.EPI-05-0658. Cancer Epidemiol Biomarkers Prev. 2006. PMID: 16537720
-
8-hydroxy-2' -deoxyguanosine (8-OHdG): A critical biomarker of oxidative stress and carcinogenesis.J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009 Apr;27(2):120-39. doi: 10.1080/10590500902885684. J Environ Sci Health C Environ Carcinog Ecotoxicol Rev. 2009. PMID: 19412858 Review.
-
The role of oxidative stress in the biological responses of lung epithelial cells to cigarette smoke.Biomarkers. 2009 Jul;14 Suppl 1:90-6. doi: 10.1080/13547500902965047. Biomarkers. 2009. PMID: 19604067 Review.
Cited by
-
Elevated urinary 8-oxo-7,8-dihydro-2'-deoxyguanosine and serum uric acid are associated with progression and are prognostic factors of colorectal cancer.Onco Targets Ther. 2018 Sep 17;11:5895-5902. doi: 10.2147/OTT.S175112. eCollection 2018. Onco Targets Ther. 2018. PMID: 30271173 Free PMC article.
-
Improvements in morphology and membrane stability obtained from TPP-TAB, a cryopreservation medium treated infertile smoker sperm cells - An in vitro study.Toxicol Rep. 2019 Aug 24;6:889-896. doi: 10.1016/j.toxrep.2019.08.015. eCollection 2019. Toxicol Rep. 2019. PMID: 31516841 Free PMC article.
-
Effect of tiger milk mushroom (Lignosus rhinocerus) supplementation on respiratory health, immunity and antioxidant status: an open-label prospective study.Sci Rep. 2021 Jun 3;11(1):11781. doi: 10.1038/s41598-021-91256-6. Sci Rep. 2021. PMID: 34083710 Free PMC article.
-
Effects of smoking cessation on biological monitoring markers in urine.Genes Environ. 2020 Sep 11;42:26. doi: 10.1186/s41021-020-00165-z. eCollection 2020. Genes Environ. 2020. PMID: 32944094 Free PMC article.
-
The impact of emphysema on surgical outcomes of early-stage lung cancer: a retrospective study.BMC Pulm Med. 2019 Apr 4;19(1):73. doi: 10.1186/s12890-019-0839-1. BMC Pulm Med. 2019. PMID: 30947705 Free PMC article.
References
-
- Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. - PubMed
-
- Wingo PA, Ries LA, Giovino GA, Miller DS, Rosenberg HM, Shopland DR, Thun MJ, Edwards BK. Annual report to the nation on the status of cancer, 1973-1996, with a special section on lung cancer and tobacco smoking. J Natl Cancer Inst. 1999;91:675–690. - PubMed
-
- Weynants P, Marchandise FX, Sibille Y. Pulmonary perspective: immunology in diagnosis and treatment of lung cancer. Eur Respir J. 1997;10:1703–1719. - PubMed
-
- Cao C, Sun SF, Lv D, Chen ZB, Ding QL, Deng ZC. Utility of VEGF and sVEGFR-1 in bronchoalveolar lavage fluid for differential diagnosis of primary lung cancer. Asian Pac J Cancer Prev. 2013;14:2443–2446. - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

