A Programmable DNA Origami Platform to Organize SNAREs for Membrane Fusion
- PMID: 26938705
- PMCID: PMC4950518
- DOI: 10.1021/jacs.5b13107
A Programmable DNA Origami Platform to Organize SNAREs for Membrane Fusion
Abstract
Soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) complexes are the core molecular machinery of membrane fusion, a fundamental process that drives inter- and intracellular communication and trafficking. One of the questions that remains controversial has been whether and how SNAREs cooperate. Here we show the use of self-assembled DNA-nanostructure rings to template uniform-sized small unilamellar vesicles containing predetermined maximal number of externally facing SNAREs to study the membrane-fusion process. We also incorporated lipid-conjugated complementary ssDNA as tethers into vesicle and target membranes, which enabled bypass of the rate-limiting docking step of fusion reactions and allowed direct observation of individual membrane-fusion events at SNARE densities as low as one pair per vesicle. With this platform, we confirmed at the single event level that, after docking of the templated-SUVs to supported lipid bilayers (SBL), one to two pairs of SNAREs are sufficient to drive fast lipid mixing. Modularity and programmability of this platform makes it readily amenable to studying more complicated systems where auxiliary proteins are involved.
Figures

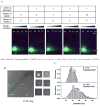
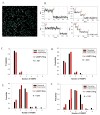
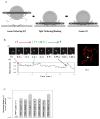

Similar articles
-
Reconstituted syntaxin1a/SNAP25 interacts with negatively charged lipids as measured by lateral diffusion in planar supported bilayers.Biophys J. 2001 Jul;81(1):266-75. doi: 10.1016/S0006-3495(01)75697-4. Biophys J. 2001. PMID: 11423412 Free PMC article.
-
Synip arrests soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE)-dependent membrane fusion as a selective target membrane SNARE-binding inhibitor.J Biol Chem. 2013 Jun 28;288(26):18885-93. doi: 10.1074/jbc.M113.465450. Epub 2013 May 12. J Biol Chem. 2013. PMID: 23665562 Free PMC article.
-
Accelerating SNARE-Mediated Membrane Fusion by DNA-Lipid Tethers.Angew Chem Int Ed Engl. 2015 Nov 23;54(48):14388-92. doi: 10.1002/anie.201506844. Epub 2015 Oct 6. Angew Chem Int Ed Engl. 2015. PMID: 26439984 Free PMC article.
-
Tethering the assembly of SNARE complexes.Trends Cell Biol. 2014 Jan;24(1):35-43. doi: 10.1016/j.tcb.2013.09.006. Epub 2013 Oct 9. Trends Cell Biol. 2014. PMID: 24119662 Review.
-
Membrane fusion in cells: molecular machinery and mechanisms.J Cell Mol Med. 2006 Apr-Jun;10(2):423-7. doi: 10.1111/j.1582-4934.2006.tb00409.x. J Cell Mol Med. 2006. PMID: 16796809 Free PMC article. Review.
Cited by
-
Single-particle combinatorial multiplexed liposome fusion mediated by DNA.Nat Chem. 2022 May;14(5):558-565. doi: 10.1038/s41557-022-00912-5. Epub 2022 Apr 4. Nat Chem. 2022. PMID: 35379901
-
Gene-encoding DNA origami for mammalian cell expression.Nat Commun. 2023 Feb 23;14(1):1017. doi: 10.1038/s41467-023-36601-1. Nat Commun. 2023. PMID: 36823187 Free PMC article.
-
Symmetrical organization of proteins under docked synaptic vesicles.FEBS Lett. 2019 Jan;593(2):144-153. doi: 10.1002/1873-3468.13316. Epub 2019 Jan 18. FEBS Lett. 2019. PMID: 30561792 Free PMC article.
-
Facile Assembly/Disassembly of DNA Nanostructures Anchored on Cell-Mimicking Giant Vesicles.J Am Chem Soc. 2017 Sep 13;139(36):12410-12413. doi: 10.1021/jacs.7b07485. Epub 2017 Aug 30. J Am Chem Soc. 2017. PMID: 28841373 Free PMC article.
-
SNARE machinery is optimized for ultrafast fusion.Proc Natl Acad Sci U S A. 2019 Feb 12;116(7):2435-2442. doi: 10.1073/pnas.1820394116. Epub 2019 Jan 30. Proc Natl Acad Sci U S A. 2019. PMID: 30700546 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

