Insight on Genes Affecting Tuber Development in Potato upon Potato spindle tuber viroid (PSTVd) Infection
- PMID: 26937634
- PMCID: PMC4777548
- DOI: 10.1371/journal.pone.0150711
Insight on Genes Affecting Tuber Development in Potato upon Potato spindle tuber viroid (PSTVd) Infection
Abstract
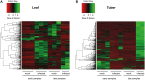
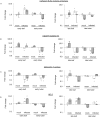
Potato (Solanum tuberosum L) is a natural host of Potato spindle tuber viroid (PSTVd) which can cause characteristic symptoms on developing plants including stunting phenotype and distortion of leaves and tubers. PSTVd is the type species of the family Pospiviroidae, and can replicate in the nucleus and move systemically throughout the plant. It is not well understood how the viroid can affect host genes for successful invasion and which genes show altered expression levels upon infection. Our primary focus in this study is the identification of genes which can affect tuber formation since viroid infection can strongly influence tuber development and especially tuber shape. In this study, we used a large-scale method to identify differentially expressed genes in potato. We have identified defence, stress and sugar metabolism related genes having altered expression levels upon infection. Additionally, hormone pathway related genes showed significant up- or down-regulation. DWARF1/DIMINUTO, Gibberellin 7-oxidase and BEL5 transcripts were identified and validated showing differential expression in viroid infected tissues. Our study suggests that gibberellin and brassinosteroid pathways have a possible role in tuber development upon PSTVd infection.
Conflict of interest statement
Figures


Similar articles
-
Silencing of transcription factor encoding gene StTCP23 by small RNAs derived from the virulence modulating region of potato spindle tuber viroid is associated with symptom development in potato.PLoS Pathog. 2019 Dec 2;15(12):e1008110. doi: 10.1371/journal.ppat.1008110. eCollection 2019 Dec. PLoS Pathog. 2019. PMID: 31790500 Free PMC article.
-
Time-Course Microarray Analysis Reveals Differences between Transcriptional Changes in Tomato Leaves Triggered by Mild and Severe Variants of Potato Spindle Tuber Viroid.Viruses. 2018 May 15;10(5):257. doi: 10.3390/v10050257. Viruses. 2018. PMID: 29762480 Free PMC article.
-
Potato Spindle Tuber Viroid Modulates Its Replication through a Direct Interaction with a Splicing Regulator.J Virol. 2018 Sep 26;92(20):e01004-18. doi: 10.1128/JVI.01004-18. Print 2018 Oct 15. J Virol. 2018. PMID: 30068655 Free PMC article.
-
Potato Spindle Tuber Viroid RNA-Templated Transcription: Factors and Regulation.Viruses. 2018 Sep 17;10(9):503. doi: 10.3390/v10090503. Viruses. 2018. PMID: 30227597 Free PMC article. Review.
-
The discovery and eradication of potato spindle tuber viroid in Canada.Virusdisease. 2014 Dec;25(4):415-24. doi: 10.1007/s13337-014-0225-9. Epub 2014 Dec 2. Virusdisease. 2014. PMID: 25674616 Free PMC article. Review.
Cited by
-
Combined analysis of the transcriptome and metabolome provides insights into the fleshy stem expansion mechanism in stem lettuce.Front Plant Sci. 2022 Dec 15;13:1101199. doi: 10.3389/fpls.2022.1101199. eCollection 2022. Front Plant Sci. 2022. PMID: 36589074 Free PMC article.
-
Transcriptomic analysis reveals insights into the response to Hop stunt viroid (HSVd) in sweet cherry (Prunus avium L.) fruits.PeerJ. 2020 Sep 21;8:e10005. doi: 10.7717/peerj.10005. eCollection 2020. PeerJ. 2020. PMID: 33005494 Free PMC article.
-
Evaluation of Disease Severity and Global Transcriptome Response Induced by Citrus bark cracking viroid, Hop latent viroid, and Their Co-Infection in Hop (Humulus lupulus L.).Int J Mol Sci. 2019 Jun 28;20(13):3154. doi: 10.3390/ijms20133154. Int J Mol Sci. 2019. PMID: 31261625 Free PMC article.
-
Revisiting the Role of Transcription Factors in Coordinating the Defense Response Against Citrus Bark Cracking Viroid Infection in Commercial Hop (Humulus Lupulus L.).Viruses. 2019 May 5;11(5):419. doi: 10.3390/v11050419. Viruses. 2019. PMID: 31060295 Free PMC article.
-
Phytohormones: plant switchers in developmental and growth stages in potato.J Genet Eng Biotechnol. 2021 Jun 17;19(1):89. doi: 10.1186/s43141-021-00192-5. J Genet Eng Biotechnol. 2021. PMID: 34142228 Free PMC article. Review.
References
-
- Flores R, Di Serio F, Navarro B, Duran-Vila N, Owens R. Viroids and Viroid Diseases of Plants In: Hurst CJ, editor. Studies in Viral Ecology: Microbial and Botanical Host Systems. Volume 1: Wiley-Blackwell; 2011. p. 307e41.
-
- Diener TO. Potato spindle tuber “virus”. Virology. 1971;45(2):411–28. - PubMed
-
- Kryczyński S, Paduch-Cichal E, Skrzeczkowski LJ. Transmission of Three Viroids Through Seed and Pollen of Tomato Plants. Journal of Phytopathology. 1988;121(1):51–7. 10.1111/j.1439-0434.1988.tb00952.x - DOI
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

