Effects of prunetin on the proteolytic activity, secretion and gene expression of MMP-3 in vitro and production of MMP-3 in vivo
- PMID: 26937219
- PMCID: PMC4770113
- DOI: 10.4196/kjpp.2016.20.2.221
Effects of prunetin on the proteolytic activity, secretion and gene expression of MMP-3 in vitro and production of MMP-3 in vivo
Abstract
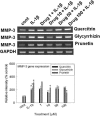
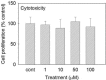
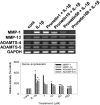
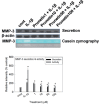
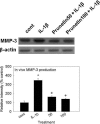
We investigated whether prunetin affects the proteolytic activity, secretion, and gene expression of matrix metalloproteinase-3 (MMP-3) in primary cultured rabbit articular chondrocytes, as well as in vivo production of MMP-3 in the rat knee joint to evaluate the potential chondroprotective eff ect of prunetin. Rabbit articular chondrocytes were cultured in a monolayer, and reverse transcription-polymerase chain reaction (RT-PCR) was used to measure interleukin-1β (IL-1β)-induced expression of MMP-3, MMP-1, MMP-13, a disintegrin and metalloproteinase with thrombospondin motifs-4 (ADAMTS-4), and ADAMTS-5. In rabbit articular chondrocytes, the effects of prunetin on IL-1β-induced secretion and proteolytic activity of MMP-3 were investigated using western blot analysis and casein zymography, respectively. The eff ect of prunetin on MMP-3 protein production was also examined in vivo. The results were as follows: (1) prunetin inhibited the gene expression of MMP-3, MMP-1, MMP-13, ADAMTS-4, and ADAMTS-5; (2) prunetin inhibited the secretion and proteolytic activity of MMP-3; (3) prunetin suppressed the production of MMP-3 protein in vivo. These results suggest that prunetin can regulate the gene expression, secretion, and proteolytic activity of MMP-3, by directly acting on articular chondrocytes.
Keywords: Chondrocyte; Metalloproteinase; Osteoarthritis; Prunetin.
Conflict of interest statement
Figures
Similar articles
-
Betulin suppressed interleukin-1β-induced gene expression, secretion and proteolytic activity of matrix metalloproteinase in cultured articular chondrocytes and production of matrix metalloproteinase in the knee joint of rat.Korean J Physiol Pharmacol. 2017 Jan;21(1):19-26. doi: 10.4196/kjpp.2017.21.1.19. Epub 2016 Dec 21. Korean J Physiol Pharmacol. 2017. PMID: 28066137 Free PMC article.
-
Apigenin Regulates Interleukin-1β-Induced Production of Matrix Metalloproteinase Both in the Knee Joint of Rat and in Primary Cultured Articular Chondrocytes.Biomol Ther (Seoul). 2016 Mar 1;24(2):163-70. doi: 10.4062/biomolther.2015.217. Biomol Ther (Seoul). 2016. PMID: 26902085 Free PMC article.
-
Effect of oleanolic acid on the activity, secretion and gene expression of matrix metalloproteinase-3 in articular chondrocytes in vitro and the production of matrix metalloproteinase-3 in vivo.Korean J Physiol Pharmacol. 2017 Mar;21(2):197-204. doi: 10.4196/kjpp.2017.21.2.197. Epub 2017 Feb 21. Korean J Physiol Pharmacol. 2017. PMID: 28280413 Free PMC article.
-
Luteolin Inhibits the Activity, Secretion and Gene Expression of MMP-3 in Cultured Articular Chondrocytes and Production of MMP-3 in the Rat Knee.Biomol Ther (Seoul). 2014 May;22(3):239-45. doi: 10.4062/biomolther.2014.020. Biomol Ther (Seoul). 2014. PMID: 25009705 Free PMC article.
-
The role of cytokines in osteoarthritis pathophysiology.Biorheology. 2002;39(1-2):237-46. Biorheology. 2002. PMID: 12082286 Review.
Cited by
-
Inhibition of the Expression of Matrix Metalloproteinases in Articular Chondrocytes by Resveratrol through Affecting Nuclear Factor-Kappa B Signaling Pathway.Biomol Ther (Seoul). 2018 Nov 1;26(6):560-567. doi: 10.4062/biomolther.2018.132. Biomol Ther (Seoul). 2018. PMID: 30464073 Free PMC article.
-
Natural Products as Sources of Novel Drug Candidates for the Pharmacological Management of Osteoarthritis: A Narrative Review.Biomol Ther (Seoul). 2019 Nov 1;27(6):503-513. doi: 10.4062/biomolther.2019.139. Biomol Ther (Seoul). 2019. PMID: 31646842 Free PMC article. Review.
-
Compound Prunetin Induces Cell Death in Gastric Cancer Cell with Potent Anti-Proliferative Properties: In Vitro Assay, Molecular Docking, Dynamics, and ADMET Studies.Biomolecules. 2020 Jul 21;10(7):1086. doi: 10.3390/biom10071086. Biomolecules. 2020. PMID: 32708333 Free PMC article.
-
Etanercept embedded silk fibroin/pullulan hydrogel enhance cartilage repair in bone marrow stimulation.Front Bioeng Biotechnol. 2022 Dec 8;10:982894. doi: 10.3389/fbioe.2022.982894. eCollection 2022. Front Bioeng Biotechnol. 2022. PMID: 36568290 Free PMC article.
-
Rosmarinic acid induces rabbit articular chondrocyte differentiation by decreases matrix metalloproteinase-13 and inflammation by upregulating cyclooxygenase-2 expression.J Biomed Sci. 2017 Sep 18;24(1):75. doi: 10.1186/s12929-017-0381-5. J Biomed Sci. 2017. PMID: 28923043 Free PMC article.
References
-
- Mankin HJ. The response of articular cartilage to mechanical injury. J Bone Joint Surg Am. 1982;64:460–466. - PubMed
-
- Kullich W, Fagerer N, Schwann H. Effect of the NSAID nimesulide on the radical scavenger glutathione S-transferase in patients with osteoarthritis of the knee. Curr Med Res Opin. 2007;23:1981–1986. - PubMed
-
- Birkedal-hansen H, Moore WG, Bodden MK, et al. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

