Autophagic and lysosomal defects in human tauopathies: analysis of post-mortem brain from patients with familial Alzheimer disease, corticobasal degeneration and progressive supranuclear palsy
- PMID: 26936765
- PMCID: PMC4774096
- DOI: 10.1186/s40478-016-0292-9
Autophagic and lysosomal defects in human tauopathies: analysis of post-mortem brain from patients with familial Alzheimer disease, corticobasal degeneration and progressive supranuclear palsy
Abstract
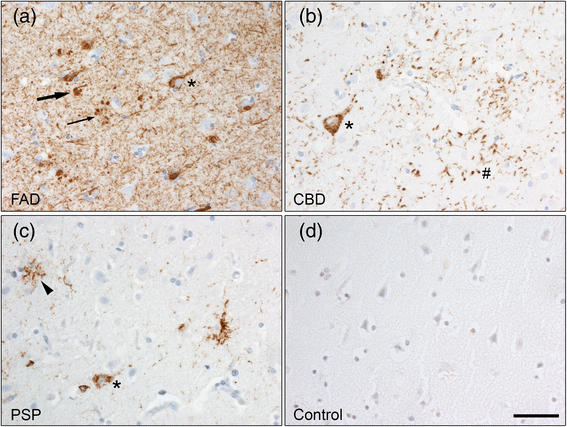
Introduction: The accumulation of insoluble proteins within neurons and glia cells is a pathological hallmark of several neurodegenerative diseases. Abnormal aggregation of the microtubule-associated protein tau characterizes the neuropathology of tauopathies, such as Alzheimer disease (AD), corticobasal degeneration (CBD) and progressive supranuclear palsy (PSP). An impairment of the lysosomal degradation pathway called macroautophagy, hereafter referred to as autophagy, could contribute to the accumulation of aggregated proteins. The role of autophagy in neurodegeneration has been intensively studied in the context of AD but there are few studies in other tauopathies and it is not known if defects in autophagy is a general feature of tauopathies. In the present study, we analysed autophagic and lysosomal markers in human post-mortem brain samples from patients with early-onset familial AD (FAD) with the APP Swedish mutation (APPswe), CBD and PSP and control individuals.
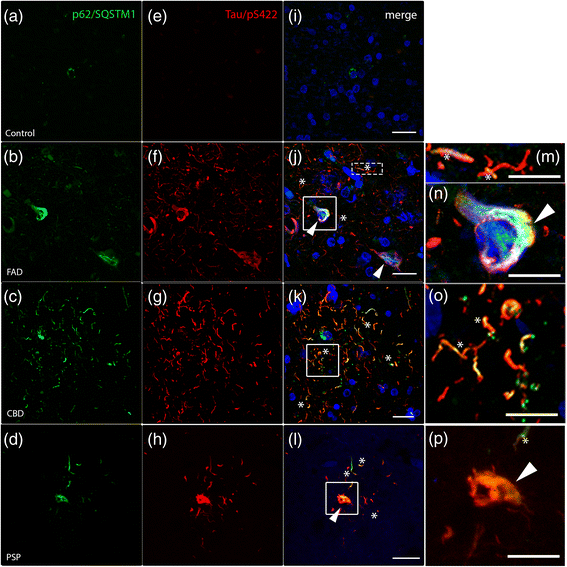
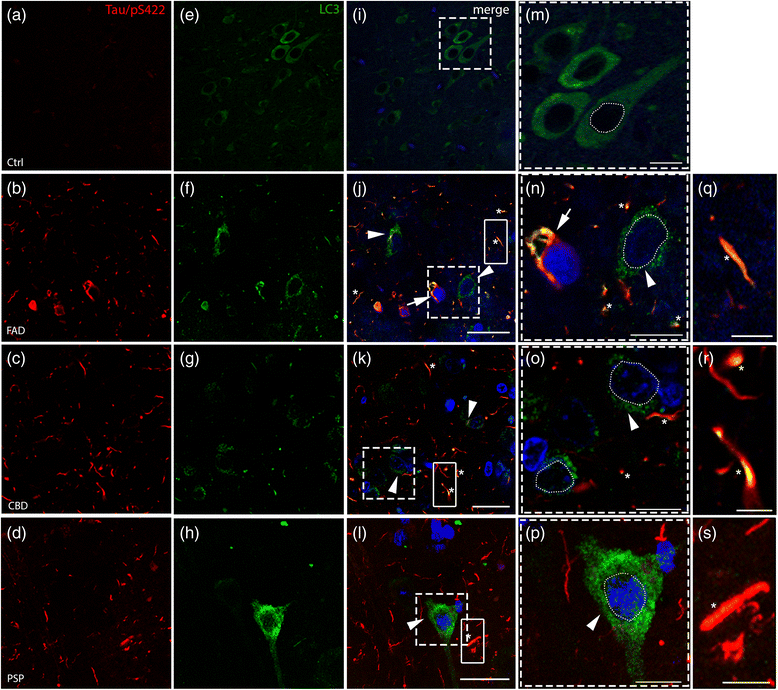
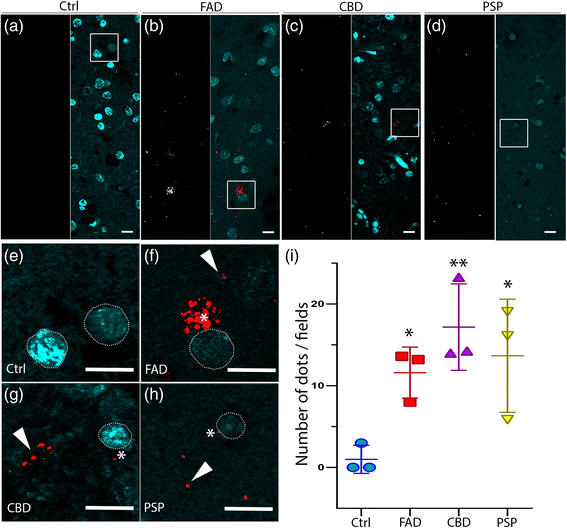
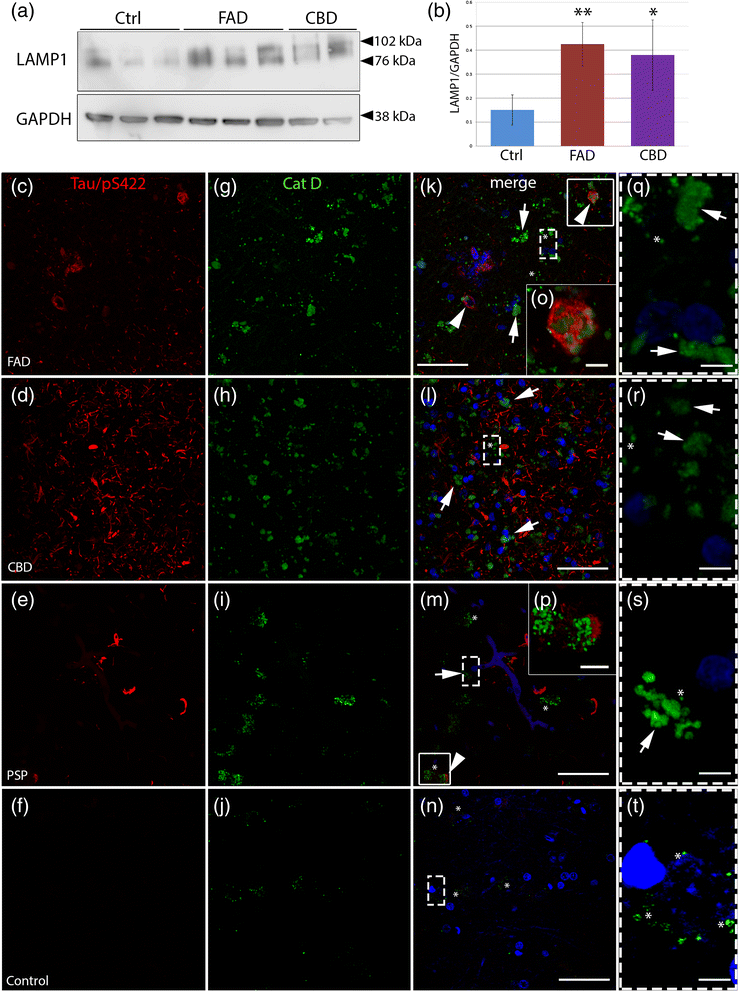
Results: FAD, CBD and PSP patients displayed an increase in LC3-positive vesicles in frontal cortex, indicating an accumulation of autophagic vesicles. Moreover, using double-immunohistochemistry and in situ proximity ligation assay, we observed colocalization of hyperphosphorylated tau with the autophagy marker LC3 in FAD, CBD and PSP patients but not in control individuals. Increased levels of the lysosomal marker LAMP1 was detected in FAD and CBD, and in addition Cathepsin D was diffusely spread in the cytoplasm in all tauopathies suggesting an impaired lysosomal integrity.
Conclusion: Taken together, our results indicate an accumulation of autophagic and lysosomal markers in human brain tissue from patients with primary tauopathies (CBD and PSP) as well as FAD, suggesting a defect of the autophagosome-lysosome pathway that may contribute to the development of tau pathology.
Figures
Similar articles
-
Decrease of neuronal FKBP4/FKBP52 modulates perinuclear lysosomal positioning and MAPT/Tau behavior during MAPT/Tau-induced proteotoxic stress.Autophagy. 2021 Nov;17(11):3491-3510. doi: 10.1080/15548627.2021.1875611. Epub 2021 Jan 25. Autophagy. 2021. PMID: 33459145 Free PMC article.
-
Phosphorylated mitogen-activated protein kinase (MAPK/ERK-P), protein kinase of 38 kDa (p38-P), stress-activated protein kinase (SAPK/JNK-P), and calcium/calmodulin-dependent kinase II (CaM kinase II) are differentially expressed in tau deposits in neurons and glial cells in tauopathies.J Neural Transm (Vienna). 2001;108(12):1397-415. doi: 10.1007/s007020100016. J Neural Transm (Vienna). 2001. PMID: 11810404
-
Phosphorylated map kinase (ERK1, ERK2) expression is associated with early tau deposition in neurones and glial cells, but not with increased nuclear DNA vulnerability and cell death, in Alzheimer disease, Pick's disease, progressive supranuclear palsy and corticobasal degeneration.Brain Pathol. 2001 Apr;11(2):144-58. doi: 10.1111/j.1750-3639.2001.tb00387.x. Brain Pathol. 2001. PMID: 11303790 Free PMC article.
-
Astrocytic inclusions in progressive supranuclear palsy and corticobasal degeneration.Neuropathology. 2014 Dec;34(6):555-70. doi: 10.1111/neup.12143. Epub 2014 Aug 14. Neuropathology. 2014. PMID: 25124031 Review.
-
Tau Interacting Proteins: Gaining Insight into the Roles of Tau in Health and Disease.Adv Exp Med Biol. 2019;1184:145-166. doi: 10.1007/978-981-32-9358-8_13. Adv Exp Med Biol. 2019. PMID: 32096036 Review.
Cited by
-
Tauopathy induced by low level expression of a human brain-derived tau fragment in mice is rescued by phenylbutyrate.Brain. 2016 Aug;139(Pt 8):2290-306. doi: 10.1093/brain/aww137. Epub 2016 Jun 12. Brain. 2016. PMID: 27297240 Free PMC article.
-
Targeting tau in Alzheimer's disease: from mechanisms to clinical therapy.Neural Regen Res. 2024 Jul 1;19(7):1489-1498. doi: 10.4103/1673-5374.385847. Epub 2023 Sep 22. Neural Regen Res. 2024. PMID: 38051891 Free PMC article.
-
The synthetic TRPML1 agonist ML-SA1 rescues Alzheimer-related alterations of the endosomal-autophagic-lysosomal system.J Cell Sci. 2023 Mar 15;136(6):jcs259875. doi: 10.1242/jcs.259875. Epub 2023 Mar 21. J Cell Sci. 2023. PMID: 36825945 Free PMC article.
-
EPPS treatment attenuates traumatic brain injury in mice by reducing Aβ burden and ameliorating neuronal autophagic flux.Exp Neurol. 2019 Apr;314:20-33. doi: 10.1016/j.expneurol.2019.01.002. Epub 2019 Jan 9. Exp Neurol. 2019. PMID: 30639321 Free PMC article.
-
Roles of Autophagy in Oxidative Stress.Int J Mol Sci. 2020 May 6;21(9):3289. doi: 10.3390/ijms21093289. Int J Mol Sci. 2020. PMID: 32384691 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

