Recombinant Lactococcus lactis expressing porcine insulin-like growth factor I ameliorates DSS-induced colitis in mice
- PMID: 26932768
- PMCID: PMC4774141
- DOI: 10.1186/s12896-016-0255-z
Recombinant Lactococcus lactis expressing porcine insulin-like growth factor I ameliorates DSS-induced colitis in mice
Abstract
Background: Insulin-like growth factor I (IGF-I) is one important family of growth factors, which plays key role in intestinal growth, regeneration, and damage repair. However, the low natural abundance of IGF-I limits its research opportunities and practical application in the fields of medicine and animal husbandry. In this study, a tandem repeat strategy was used to express three copies of the same pIGF-I3 protein in L. lactis. The activity of recombinant pIGF-I3 (rpIGF-I3) was further examined by a mouse model of dextran sulfate sodium (DSS)-induced colitis. In addition, the potential of recombinant L. lactis expressing pIGF-I3 to reduce inflammatory disease was evaluated.
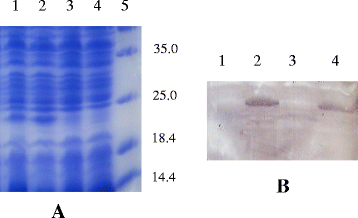
Results: pIGF-I3 could be expressed in L. lactis by the detection of SDS-PAGE and Western blot. Experimental colitis was induced in BALB/c mice by administration of 5 % DSS in drinking water, and the clinical symptoms were observed in DSS-treated mice. Oral administration of recombinant L. lactis expressing pIGF-I3 improved the colonic architecture, and significantly reduced the increase of colonic damage score (P < 0.05). Furthermore, recombinant L. lactis expressing pIGF-I3 treatment significantly reduced serum DAO activity and colonic MPO level, and elevated colonic occludin level compared to the DSS group (P < 0.05).
Conclusions: The pIGF-I3 expressed in L. lactis has good biological activity, and oral administration of recombinant L. lactis expressing pIGF-I3 attenuated the symptoms and development of DSS-induced colitis in mice. These suggested that L. lactis could be a potential host bacterium for production and delivery of IGF-I against intestinal diseases.
Figures
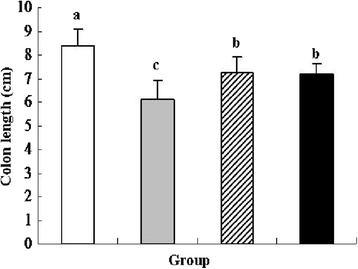
 ) received normal drinking water. Group 2 (DSS
) received normal drinking water. Group 2 (DSS  ) received DSS solution in drinking water. Group 3 (L. lactis
) received DSS solution in drinking water. Group 3 (L. lactis
 ) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148). Group 4 (recombinant L. lactis
) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148). Group 4 (recombinant L. lactis
 ) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148- pIGF-I3). Same letters between the groups indicate no statistically significant difference (P > 0.05), and different letters indicate statistically significant difference (P < 0.05)
) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148- pIGF-I3). Same letters between the groups indicate no statistically significant difference (P > 0.05), and different letters indicate statistically significant difference (P < 0.05)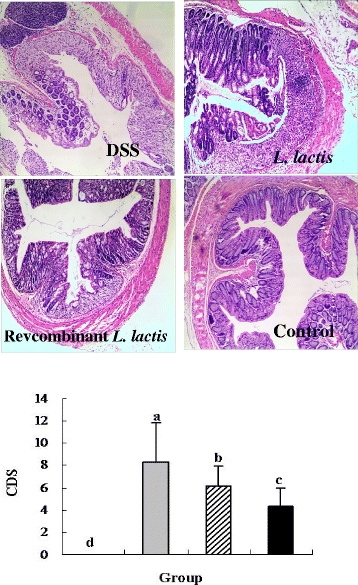
 ) received normal drinking water. Group 2 (DSS
) received normal drinking water. Group 2 (DSS  ) received DSS solution in drinking water. Group 3 (L. lactis
) received DSS solution in drinking water. Group 3 (L. lactis
 ) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148). Group 4 (recombinant L. lactis
) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148). Group 4 (recombinant L. lactis
 ) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148-pIGF-I3). Same letters between the groups indicate no statistically significant difference (P > 0.05), and different letters indicate statistically significant difference (P < 0.05)
) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148-pIGF-I3). Same letters between the groups indicate no statistically significant difference (P > 0.05), and different letters indicate statistically significant difference (P < 0.05)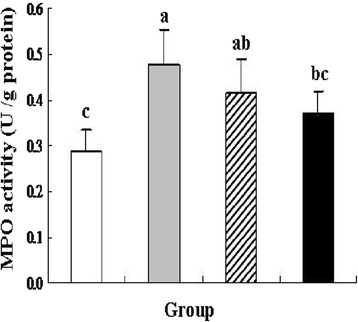
 ) received normal drinking water. Group 2 (DSS
) received normal drinking water. Group 2 (DSS  ) received DSS solution in drinking water. Group 3 (L. lactis
) received DSS solution in drinking water. Group 3 (L. lactis
 ) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148). Group 4 (recombinant L. lactis
) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148). Group 4 (recombinant L. lactis
 ) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148-pIGF-I3). Same letters between the groups indicate no statistically significant difference (P > 0.05), and different letters indicate statistically significant difference (P < 0.05)
) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148-pIGF-I3). Same letters between the groups indicate no statistically significant difference (P > 0.05), and different letters indicate statistically significant difference (P < 0.05)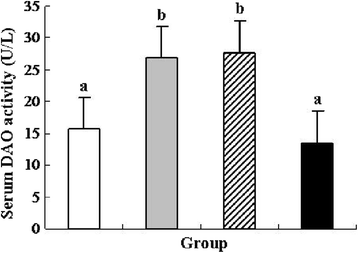
 ) received normal drinking water. Group 2 (DSS
) received normal drinking water. Group 2 (DSS  ) received DSS solution in drinking water. Group 3 (L. lactis
) received DSS solution in drinking water. Group 3 (L. lactis
 ) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148). Group 4 (recombinant L. lactis
) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148). Group 4 (recombinant L. lactis
 ) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148-pIGF-I3). Same letters between the groups indicate no statistically significant difference (P > 0.05), and different letters indicate statistically significant difference (P < 0.05)
) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148-pIGF-I3). Same letters between the groups indicate no statistically significant difference (P > 0.05), and different letters indicate statistically significant difference (P < 0.05)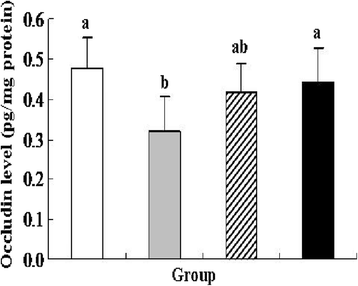
 ) received normal drinking water. Group 2 (DSS
) received normal drinking water. Group 2 (DSS  ) received DSS solution in drinking water. Group 3 (L. lactis
) received DSS solution in drinking water. Group 3 (L. lactis
 ) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148). Group 4 (recombinant L. lactis
) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148). Group 4 (recombinant L. lactis
 ) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148-pIGF-I3). Same letters between the groups indicate no statistically significant difference (P > 0.05), and different letters indicate statistically significant difference (P < 0.05)
) received the same treatment of DSS as Group 2. Meanwhile, these mice orally administered with L. lactis NZ9000 (pNZ8148-pIGF-I3). Same letters between the groups indicate no statistically significant difference (P > 0.05), and different letters indicate statistically significant difference (P < 0.05)Similar articles
-
Prevention and treatment of colitis with Lactococcus lactis secreting the immunomodulatory Yersinia LcrV protein.Gastroenterology. 2007 Sep;133(3):862-74. doi: 10.1053/j.gastro.2007.06.018. Epub 2007 Jun 20. Gastroenterology. 2007. PMID: 17678918
-
Preventative delivery of IL-35 by Lactococcus lactis ameliorates DSS-induced colitis in mice.Appl Microbiol Biotechnol. 2019 Oct;103(19):7931-7941. doi: 10.1007/s00253-019-10094-9. Epub 2019 Aug 27. Appl Microbiol Biotechnol. 2019. PMID: 31456001
-
Fasting prevents experimental murine colitis produced by dextran sulfate sodium and decreases interleukin-1 beta and insulin-like growth factor I messenger ribonucleic acid.Endocrinology. 1997 Feb;138(2):734-40. doi: 10.1210/endo.138.2.4941. Endocrinology. 1997. PMID: 9003009
-
Dextran sulphate sodium colitis in C57BL/6J mice is alleviated by Lactococcus lactis and worsened by the neutralization of Tumor necrosis Factor α.Int Immunopharmacol. 2017 Feb;43:219-226. doi: 10.1016/j.intimp.2016.12.027. Epub 2016 Dec 29. Int Immunopharmacol. 2017. PMID: 28039805
-
Delivery of therapeutic proteins through Lactococcus lactis.Biotechnol Genet Eng Rev. 2006;22:253-66. doi: 10.1080/02648725.2006.10648073. Biotechnol Genet Eng Rev. 2006. PMID: 18476334 Review. No abstract available.
Cited by
-
Recombinant Lactococcus Expressing a Novel Variant of Infectious Bursal Disease Virus VP2 Protein Can Induce Unique Specific Neutralizing Antibodies in Chickens and Provide Complete Protection.Viruses. 2020 Nov 25;12(12):1350. doi: 10.3390/v12121350. Viruses. 2020. PMID: 33255742 Free PMC article.
-
Secretion of tumoricidal human tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) by recombinant Lactococcus lactis: optimization of in vitro synthesis conditions.Microb Cell Fact. 2018 Nov 16;17(1):177. doi: 10.1186/s12934-018-1028-2. Microb Cell Fact. 2018. PMID: 30446013 Free PMC article.
-
The Anti-Tumor Effect of Lactococcus lactis Bacteria-Secreting Human Soluble TRAIL Can Be Enhanced by Metformin Both In Vitro and In Vivo in a Mouse Model of Human Colorectal Cancer.Cancers (Basel). 2021 Jun 15;13(12):3004. doi: 10.3390/cancers13123004. Cancers (Basel). 2021. PMID: 34203951 Free PMC article.
-
Plasmid Replicons for the Production of Pharmaceutical-Grade pDNA, Proteins and Antigens by Lactococcus lactis Cell Factories.Int J Mol Sci. 2021 Jan 30;22(3):1379. doi: 10.3390/ijms22031379. Int J Mol Sci. 2021. PMID: 33573129 Free PMC article. Review.
-
PVA enema ameliorates DSS-induced acute colitis in mice.BMC Gastroenterol. 2023 Oct 30;23(1):368. doi: 10.1186/s12876-023-03005-w. BMC Gastroenterol. 2023. PMID: 37904100 Free PMC article.
References
-
- Tavakkol A, Simmen FA, Simmen RC. Porcine insulin-like growth factor-I (pIGF-I): complementary deoxyribonucleic acid cloning and uterine expression of messenger ribonucleic acid encoding evolutionarily conserved IGF-I peptides. Mol Endocrinol. 1988;2:674–81. doi: 10.1210/mend-2-8-674. - DOI - PubMed
-
- Chapman IM, Hartman ML, Pieper KS, Skiles EH, Pezzoli SS, Hintz RL, et al. Recovery of growth hormone release from suppression by exogenous insulin-like growth factor I (IGF-I): evidence for a suppressive action of free rather than bound IGF-I. J Clin Endocrinol Metab. 1998;83:2836–42. - PubMed
-
- Li X, Yin J, Li D, Chen X, Zang J, Zhou X. Dietary supplementation with zinc oxide increases Igf-I and Igf-I receptor gene expression in the small intestine of weanling piglets. J Nutr. 2006;136:1786–91. - PubMed
-
- Laburthe M, Rouyer-Fessard C, Gammeltoft S. Receptors for insulin-like growth factors I and II in rat gastrointestinal epithelium. Am J Physiol. 1988;254(3 Pt 1):G457–62. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

