Stereocilia-staircase spacing is influenced by myosin III motors and their cargos espin-1 and espin-like
- PMID: 26926603
- PMCID: PMC4773517
- DOI: 10.1038/ncomms10833
Stereocilia-staircase spacing is influenced by myosin III motors and their cargos espin-1 and espin-like
Erratum in
-
Corrigendum: Stereocilia-staircase spacing is influenced by myosin III motors and their cargos espin-1 and espin-like.Nat Commun. 2017 Aug 14;8:16133. doi: 10.1038/ncomms16133. Nat Commun. 2017. PMID: 28805204 Free PMC article.
Abstract
Hair cells tightly control the dimensions of their stereocilia, which are actin-rich protrusions with graded heights that mediate mechanotransduction in the inner ear. Two members of the myosin-III family, MYO3A and MYO3B, are thought to regulate stereocilia length by transporting cargos that control actin polymerization at stereocilia tips. We show that eliminating espin-1 (ESPN-1), an isoform of ESPN and a myosin-III cargo, dramatically alters the slope of the stereocilia staircase in a subset of hair cells. Furthermore, we show that espin-like (ESPNL), primarily present in developing stereocilia, is also a myosin-III cargo and is essential for normal hearing. ESPN-1 and ESPNL each bind MYO3A and MYO3B, but differentially influence how the two motors function. Consequently, functional properties of different motor-cargo combinations differentially affect molecular transport and the length of actin protrusions. This mechanism is used by hair cells to establish the required range of stereocilia lengths within a single cell.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
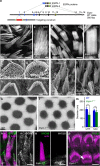
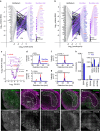
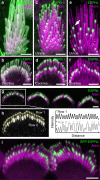
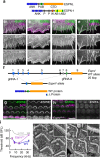
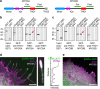
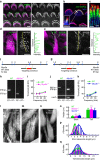
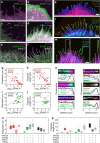
Similar articles
-
Myosin IIIB uses an actin-binding motif in its espin-1 cargo to reach the tips of actin protrusions.Curr Biol. 2012 Feb 21;22(4):320-5. doi: 10.1016/j.cub.2011.12.053. Epub 2012 Jan 19. Curr Biol. 2012. PMID: 22264607 Free PMC article.
-
Competition and compensation: dissecting the biophysical and functional differences between the class 3 myosin paralogs, myosins 3a and 3b.Bioarchitecture. 2012 Sep-Oct;2(5):171-4. doi: 10.4161/bioa.21733. Epub 2012 Sep 1. Bioarchitecture. 2012. PMID: 22954581 Free PMC article.
-
Class III myosins shape the auditory hair bundles by limiting microvilli and stereocilia growth.J Cell Biol. 2016 Jan 18;212(2):231-44. doi: 10.1083/jcb.201509017. Epub 2016 Jan 11. J Cell Biol. 2016. PMID: 26754646 Free PMC article.
-
Functional Role of Class III Myosins in Hair Cells.Front Cell Dev Biol. 2021 Feb 25;9:643856. doi: 10.3389/fcell.2021.643856. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33718386 Free PMC article. Review.
-
Human deafness-associated variants alter the dynamics of key molecules in hair cell stereocilia F-actin cores.Hum Genet. 2022 Apr;141(3-4):363-382. doi: 10.1007/s00439-021-02304-0. Epub 2021 Jul 7. Hum Genet. 2022. PMID: 34232383 Free PMC article. Review.
Cited by
-
Differential regulation of hair cell actin cytoskeleton mediated by SRF and MRTFB.Elife. 2023 Nov 20;12:e90155. doi: 10.7554/eLife.90155. Elife. 2023. PMID: 37982489 Free PMC article.
-
Consistent DNA Hypomethylations in Prostate Cancer.Int J Mol Sci. 2022 Dec 26;24(1):386. doi: 10.3390/ijms24010386. Int J Mol Sci. 2022. PMID: 36613831 Free PMC article.
-
Protrusion growth driven by myosin-generated force.Dev Cell. 2023 Jan 9;58(1):18-33.e6. doi: 10.1016/j.devcel.2022.12.001. Dev Cell. 2023. PMID: 36626869 Free PMC article.
-
Single-cell RNA-sequencing analysis of the developing mouse inner ear identifies molecular logic of auditory neuron diversification.Nat Commun. 2022 Jul 5;13(1):3878. doi: 10.1038/s41467-022-31580-1. Nat Commun. 2022. PMID: 35790771 Free PMC article.
-
Mechanotransduction current is essential for stability of the transducing stereocilia in mammalian auditory hair cells.Elife. 2017 Mar 28;6:e24661. doi: 10.7554/eLife.24661. Elife. 2017. PMID: 28350294 Free PMC article.
References
-
- Mattila P. K. & Lappalainen P. Filopodia: molecular architecture and cellular functions. Nat. Rev. Mol. Cell Biol. 9, 446–454 (2008). - PubMed
-
- Tilney L. G., Tilney M. S. & DeRosier D. J. Actin filaments, stereocilia, and hair cells: how cells count and measure. Annu. Rev. Cell Biol. 8, 257–274 (1992). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

