NanoLuc Luciferase - A Multifunctional Tool for High Throughput Antibody Screening
- PMID: 26924984
- PMCID: PMC4758271
- DOI: 10.3389/fphar.2016.00027
NanoLuc Luciferase - A Multifunctional Tool for High Throughput Antibody Screening
Abstract
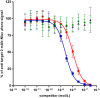
Based on the recent development of NanoLuc luciferase (Nluc), a small (19 kDa), highly stable, ATP independent, bioluminescent protein, an extremely robust and ultra high sensitivity screening system has been developed whereby primary hits of therapeutic antibodies and antibody fragments could be characterized and quantified without purification. This system is very versatile allowing cellular and solid phase ELISA but also homogeneous BRET based screening assays, relative affinity determinations with competition ELISA and direct Western blotting. The new Nluc protein fusion represents a "swiss army knife solution" for today and future high throughput antibody drug screenings.
Keywords: NanoLuc®; Western blot; affinity determination; bioluminescence resonance energy transfer (BRET); expression analysis; homogeneous assay; library screening; luciferase; phage display.
Figures


Similar articles
-
New Horizons on Molecular Pharmacology Applied to Drug Discovery: When Resonance Overcomes Radioligand Binding.Curr Radiopharm. 2017;10(1):16-20. doi: 10.2174/1874471010666170208152420. Curr Radiopharm. 2017. PMID: 28183248 Review.
-
A slow but steady nanoLuc: R162A mutation results in a decreased, but stable, nanoLuc activity.Int J Biol Macromol. 2024 Jun;269(Pt 1):131864. doi: 10.1016/j.ijbiomac.2024.131864. Epub 2024 Apr 29. Int J Biol Macromol. 2024. PMID: 38692549
-
NanoLuc luciferase as a suitable fusion partner of recombinant antibody fragments for developing sensitive luminescent immunoassays.Anal Chim Acta. 2021 May 29;1161:238180. doi: 10.1016/j.aca.2020.12.055. Epub 2021 Jan 6. Anal Chim Acta. 2021. PMID: 33896564
-
Ratiometric BRET Measurements of ATP with a Genetically-Encoded Luminescent Sensor.Sensors (Basel). 2019 Aug 10;19(16):3502. doi: 10.3390/s19163502. Sensors (Basel). 2019. PMID: 31405152 Free PMC article.
-
NanoBRET: The Bright Future of Proximity-Based Assays.Front Bioeng Biotechnol. 2019 Mar 26;7:56. doi: 10.3389/fbioe.2019.00056. eCollection 2019. Front Bioeng Biotechnol. 2019. PMID: 30972335 Free PMC article. Review.
Cited by
-
Engineering and exploiting synthetic allostery of NanoLuc luciferase.Nat Commun. 2022 Feb 10;13(1):789. doi: 10.1038/s41467-022-28425-2. Nat Commun. 2022. PMID: 35145068 Free PMC article.
-
Dual-Color Bioluminescent Sensor Proteins for Therapeutic Drug Monitoring of Antitumor Antibodies.Anal Chem. 2018 Mar 6;90(5):3592-3599. doi: 10.1021/acs.analchem.8b00041. Epub 2018 Feb 22. Anal Chem. 2018. PMID: 29443503 Free PMC article.
-
DNA Engineering and Hepatitis B Virus Replication.Front Microbiol. 2021 Nov 11;12:783040. doi: 10.3389/fmicb.2021.783040. eCollection 2021. Front Microbiol. 2021. PMID: 34858381 Free PMC article.
-
The right tools for the job: the central role for next generation chemical probes and chemistry-based target deconvolution methods in phenotypic drug discovery.RSC Med Chem. 2021 Mar 24;12(5):646-665. doi: 10.1039/d1md00022e. RSC Med Chem. 2021. PMID: 34124668 Free PMC article. Review.
-
Bioluminescent Antibodies through Photoconjugation of Protein G-Luciferase Fusion Proteins.Bioconjug Chem. 2020 Mar 18;31(3):656-662. doi: 10.1021/acs.bioconjchem.9b00804. Epub 2020 Jan 22. Bioconjug Chem. 2020. PMID: 31909607 Free PMC article.
References
-
- Boute N., Pernet K., Issad T. (2001). Monitoring the activation state of the insulin receptor using bioluminescence resonance energy transfer. Mol. Pharmacol. 60 640–645. - PubMed
-
- Cassimeris L., Guglielmi L., Denis V., Larroque C., Martineau P. (2013). Specific in vivo labeling of tyrosinated alpha-tubulin and measurement of microtubule dynamics using a GFP tagged, cytoplasmically expressed recombinant antibody. PLoS ONE 8:e59812 10.1371/journal.pone.0059812 - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources

