Apigenin Regulates Interleukin-1β-Induced Production of Matrix Metalloproteinase Both in the Knee Joint of Rat and in Primary Cultured Articular Chondrocytes
- PMID: 26902085
- PMCID: PMC4774497
- DOI: 10.4062/biomolther.2015.217
Apigenin Regulates Interleukin-1β-Induced Production of Matrix Metalloproteinase Both in the Knee Joint of Rat and in Primary Cultured Articular Chondrocytes
Abstract
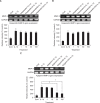
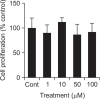
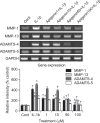
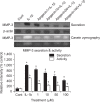
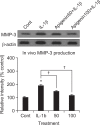
We examined whether apigenin affects the gene expression, secretion and activity of matrix metalloproteinase-3 (MMP-3) in primary cultured rabbit articular chondrocytes, as well as in vivo production of MMP-3 in the knee joint of rat to evaluate the potential chondroprotective effects of apigenin. Rabbit articular chondrocytes were cultured in a monolayer, and reverse transcription - polymerase chain reaction (RT-PCR) was used to measure interleukin-1β (IL-1β)-induced expression of MMP-3, MMP-1, MMP-13, a disintegrin and metalloproteinase with thrombospondin motifs-4 (ADAMTS-4), and ADAMTS-5. In rabbit articular chondrocytes, the effects of apigenin on IL-1β-induced secretion and proteolytic activity of MMP-3 were investigated using western blot analysis and casein zymography, respectively. The effect of apigenin on MMP-3 protein production was also examined in vivo. In rabbit articular chondrocytes, apigenin inhibited the gene expression of MMP-3, MMP-1, MMP-13, ADAMTS-4, and ADAMTS-5. Furthermore, apigenin inhibited the secretion and proteolytic activity of MMP-3 in vitro, and inhibited production of MMP-3 protein in vivo. These results suggest that apigenin can regulate the gene expression, secretion, and activity of MMP-3, by directly acting on articular chondrocytes.
Keywords: Apigenin; Chondrocyte; Metalloproteinase; Osteoarthritis.
Figures
Similar articles
-
Betulin suppressed interleukin-1β-induced gene expression, secretion and proteolytic activity of matrix metalloproteinase in cultured articular chondrocytes and production of matrix metalloproteinase in the knee joint of rat.Korean J Physiol Pharmacol. 2017 Jan;21(1):19-26. doi: 10.4196/kjpp.2017.21.1.19. Epub 2016 Dec 21. Korean J Physiol Pharmacol. 2017. PMID: 28066137 Free PMC article.
-
Effects of prunetin on the proteolytic activity, secretion and gene expression of MMP-3 in vitro and production of MMP-3 in vivo.Korean J Physiol Pharmacol. 2016 Mar;20(2):221-8. doi: 10.4196/kjpp.2016.20.2.221. Epub 2016 Feb 23. Korean J Physiol Pharmacol. 2016. PMID: 26937219 Free PMC article.
-
Effect of oleanolic acid on the activity, secretion and gene expression of matrix metalloproteinase-3 in articular chondrocytes in vitro and the production of matrix metalloproteinase-3 in vivo.Korean J Physiol Pharmacol. 2017 Mar;21(2):197-204. doi: 10.4196/kjpp.2017.21.2.197. Epub 2017 Feb 21. Korean J Physiol Pharmacol. 2017. PMID: 28280413 Free PMC article.
-
Chondroprotective Effects of Wogonin in Experimental Models of Osteoarthritis in vitro and in vivo.Biomol Ther (Seoul). 2015 Sep;23(5):442-8. doi: 10.4062/biomolther.2015.045. Epub 2015 Sep 1. Biomol Ther (Seoul). 2015. PMID: 26336584 Free PMC article.
-
Luteolin Inhibits the Activity, Secretion and Gene Expression of MMP-3 in Cultured Articular Chondrocytes and Production of MMP-3 in the Rat Knee.Biomol Ther (Seoul). 2014 May;22(3):239-45. doi: 10.4062/biomolther.2014.020. Biomol Ther (Seoul). 2014. PMID: 25009705 Free PMC article.
Cited by
-
Betulin suppressed interleukin-1β-induced gene expression, secretion and proteolytic activity of matrix metalloproteinase in cultured articular chondrocytes and production of matrix metalloproteinase in the knee joint of rat.Korean J Physiol Pharmacol. 2017 Jan;21(1):19-26. doi: 10.4196/kjpp.2017.21.1.19. Epub 2016 Dec 21. Korean J Physiol Pharmacol. 2017. PMID: 28066137 Free PMC article.
-
Ononin ameliorates inflammation and cartilage degradation in rat chondrocytes with IL-1β-induced osteoarthritis by downregulating the MAPK and NF-κB pathways.BMC Complement Med Ther. 2022 Jan 27;22(1):25. doi: 10.1186/s12906-022-03504-5. BMC Complement Med Ther. 2022. PMID: 35086536 Free PMC article.
-
Efficacy of Combination Therapy with Apigenin and Synovial Membrane-Derived Mesenchymal Stem Cells on Knee Joint Osteoarthritis in a Rat Model.Iran J Med Sci. 2021 Sep;46(5):383-394. doi: 10.30476/IJMS.2020.83686.1301. Iran J Med Sci. 2021. PMID: 34539013 Free PMC article.
-
Comparison of diclofenac with apigenin-glycosides rich Clinacanthus nutans extract for amending inflammation and catabolic protease regulations in osteoporotic-osteoarthritis rat model.Daru. 2020 Dec;28(2):443-453. doi: 10.1007/s40199-020-00343-y. Epub 2020 May 9. Daru. 2020. PMID: 32388789 Free PMC article.
-
Natural Compounds: Potential Therapeutics for the Inhibition of Cartilage Matrix Degradation in Osteoarthritis.Life (Basel). 2022 Dec 30;13(1):102. doi: 10.3390/life13010102. Life (Basel). 2022. PMID: 36676051 Free PMC article. Review.
References
-
- Birkedal-Hansen H, Moore WG, Bodden MK, Windsor LJ, Birkedal-Hansen B, DeCarlo A, Engler JA. Matrix metalloproteinases: a review. Crit Rev Oral Biol Med. 1993;4:197–250. - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

