Podoplanin is a component of extracellular vesicles that reprograms cell-derived exosomal proteins and modulates lymphatic vessel formation
- PMID: 26893367
- PMCID: PMC4941298
- DOI: 10.18632/oncotarget.7445
Podoplanin is a component of extracellular vesicles that reprograms cell-derived exosomal proteins and modulates lymphatic vessel formation
Abstract
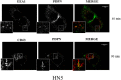
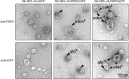
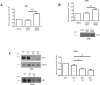
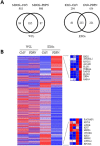
Podoplanin (PDPN) is a transmembrane glycoprotein that plays crucial roles in embryonic development, the immune response, and malignant progression. Here, we report that cells ectopically or endogenously expressing PDPN release extracellular vesicles (EVs) that contain PDPN mRNA and protein. PDPN incorporates into membrane shed microvesicles (MVs) and endosomal-derived exosomes (EXOs), where it was found to colocalize with the canonical EV marker CD63 by immunoelectron microscopy. We have previously found that expression of PDPN in MDCK cells induces an epithelial-mesenchymal transition (EMT). Proteomic profiling of MDCK-PDPN cells compared to control cells shows that PDPN-induced EMT is associated with upregulation of oncogenic proteins and diminished expression of tumor suppressors. Proteomic analysis of exosomes reveals that MDCK-PDPN EXOs were enriched in protein cargos involved in cell adhesion, cytoskeletal remodeling, signal transduction and, importantly, intracellular trafficking and EV biogenesis. Indeed, expression of PDPN in MDCK cells stimulated both EXO and MV production, while knockdown of endogenous PDPN in human HN5 squamous carcinoma cells reduced EXO production and inhibited tumorigenesis. EXOs released from MDCK-PDPN and control cells both stimulated in vitro angiogenesis, but only EXOs containing PDPN were shown to promote lymphatic vessel formation. This effect was mediated by PDPN on the surface of EXOs, as demonstrated by a neutralizing specific monoclonal antibody. These results contribute to our understanding of PDPN-induced EMT in association to tumor progression, and suggest an important role for PDPN in EV biogenesis and/or release and for PDPN-EXOs in modulating lymphangiogenesis.
Keywords: exosomes; lymphangiogenesis; microvesicles; podoplanin; tumor progression.
Conflict of interest statement
The authors declare no conflict of interest.
Figures




Similar articles
-
Oncogenic H-ras reprograms Madin-Darby canine kidney (MDCK) cell-derived exosomal proteins following epithelial-mesenchymal transition.Mol Cell Proteomics. 2013 Aug;12(8):2148-59. doi: 10.1074/mcp.M112.027086. Epub 2013 May 3. Mol Cell Proteomics. 2013. PMID: 23645497 Free PMC article.
-
Oncogenic epithelial cell-derived exosomes containing Rac1 and PAK2 induce angiogenesis in recipient endothelial cells.Oncotarget. 2016 Apr 12;7(15):19709-22. doi: 10.18632/oncotarget.7573. Oncotarget. 2016. PMID: 26919098 Free PMC article.
-
Highly-purified exosomes and shed microvesicles isolated from the human colon cancer cell line LIM1863 by sequential centrifugal ultrafiltration are biochemically and functionally distinct.Methods. 2015 Oct 1;87:11-25. doi: 10.1016/j.ymeth.2015.04.008. Epub 2015 Apr 16. Methods. 2015. PMID: 25890246
-
Roles of Podoplanin in Malignant Progression of Tumor.Cells. 2022 Feb 7;11(3):575. doi: 10.3390/cells11030575. Cells. 2022. PMID: 35159384 Free PMC article. Review.
-
Emerging roles of exosomes during epithelial-mesenchymal transition and cancer progression.Semin Cell Dev Biol. 2015 Apr;40:60-71. doi: 10.1016/j.semcdb.2015.02.008. Epub 2015 Feb 23. Semin Cell Dev Biol. 2015. PMID: 25721809 Review.
Cited by
-
Reprogramming of sentinel lymph node microenvironment during tumor metastasis.J Biomed Sci. 2022 Oct 20;29(1):84. doi: 10.1186/s12929-022-00868-1. J Biomed Sci. 2022. PMID: 36266717 Free PMC article. Review.
-
Small Extracellular Vesicles in Transplant Rejection.Cells. 2021 Nov 3;10(11):2989. doi: 10.3390/cells10112989. Cells. 2021. PMID: 34831212 Free PMC article. Review.
-
Extracellular Vesicles Linking Inflammation, Cancer and Thrombotic Risks.Front Cell Dev Biol. 2022 Mar 17;10:859863. doi: 10.3389/fcell.2022.859863. eCollection 2022. Front Cell Dev Biol. 2022. PMID: 35372327 Free PMC article. Review.
-
Levels of human proteins in plasma associated with acute paediatric malaria.Malar J. 2018 Nov 15;17(1):426. doi: 10.1186/s12936-018-2576-y. Malar J. 2018. PMID: 30442134 Free PMC article.
-
Treatment of Oxidative Stress with Exosomes in Myocardial Ischemia.Int J Mol Sci. 2021 Feb 9;22(4):1729. doi: 10.3390/ijms22041729. Int J Mol Sci. 2021. PMID: 33572188 Free PMC article. Review.
References
-
- Renart J, Carrasco-Ramirez P, Fernandez-Munoz B, Martin-Villar E, Montero L, Yurrita MM, Quintanilla M. New insights into the role of podoplanin in epithelial-mesenchymal transition. Int Rev Cell Mol Biol. 2015;317:185–239. - PubMed
-
- Martin-Villar E, Scholl FG, Gamallo C, Yurrita MM, Munoz-Guerra M, Cruces J, Quintanilla M. Characterization of human PA2.26 antigen (T1alpha-2, podoplanin), a small membrane mucin induced in oral squamous cell carcinomas. Int J Cancer. 2005;113:899–910. - PubMed
-
- Wicki A, Lehembre F, Wick N, Hantusch B, Kerjaschki D, Christofori G. Tumor invasion in the absence of epithelial-mesenchymal transition: podoplanin-mediated remodeling of the actin cytoskeleton. Cancer Cell. 2006;9:261–272. - PubMed
-
- Yuan P, Temam S, El-Naggar A, Zhou X, Liu DD, Lee JJ, Mao L. Overexpression of podoplanin in oral cancer and its association with poor clinical outcome. Cancer. 2006;107:563–569. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

