The non-Geldanamycin Hsp90 inhibitors enhanced the antifungal activity of fluconazole
- PMID: 26885259
- PMCID: PMC4731659
The non-Geldanamycin Hsp90 inhibitors enhanced the antifungal activity of fluconazole
Abstract
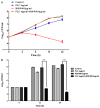
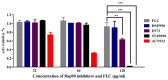
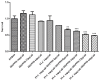
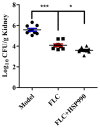
The molecular chaperone heat shock protein 90 (Hsp90) is highly conserved in eukaryotes and facilitates the correct folding, productive assembly and maturation of a diverse cellular proteins. In fungi, especially the most prevalent human fungal pathogen Candida albicans, Hsp90 influences development and modulates drug resistance. Here, we mainly explore the effect of non-Geldanamycin Hsp90 inhibitor HSP990 on the activity of fluconazole (FLC) against Candida albicans and investigate the underlying mechanism. We demonstrate that HSP990 has potent synergistic antifungal activity with FLC against FLC-resistant C. albicans through the checkerboard microdilution assay,agar diffusion tests and time-kill curves, and shows low cytotoxicity to human umbilical vein endothelial cells. Further study shows that the activity of FLC against C. albicans biofilm formation in vitro is significantly enhanced when used in combination with HSP990. In a murine model of disseminated candidiasis, the therapeutic efficacy of FLC is also enhanced by the pharmacological inhibition of C. albicans Hsp90 function with HSP990. Thus, the combined use of small molecule compound and existing antifungal drugs may provide a potential therapeutic strategy for fungal infectious disease.
Keywords: Hsp90 inhibitors; fluconazole; fungal pathogens; synergistic antifungal activity.
Figures


Similar articles
-
The Synergism of the Small Molecule ENOblock and Fluconazole Against Fluconazole-Resistant Candida albicans.Front Microbiol. 2019 Sep 6;10:2071. doi: 10.3389/fmicb.2019.02071. eCollection 2019. Front Microbiol. 2019. PMID: 31555252 Free PMC article.
-
Antifungal activity of geldanamycin alone or in combination with fluconazole against Candida species.Mycopathologia. 2013 Apr;175(3-4):273-9. doi: 10.1007/s11046-012-9612-1. Epub 2013 Jan 23. Mycopathologia. 2013. PMID: 23341047
-
Synergistic antifungal activity of berberine derivative B-7b and fluconazole.PLoS One. 2015 May 19;10(5):e0126393. doi: 10.1371/journal.pone.0126393. eCollection 2015. PLoS One. 2015. PMID: 25992630 Free PMC article.
-
Combination of fluconazole with non-antifungal agents: a promising approach to cope with resistant Candida albicans infections and insight into new antifungal agent discovery.Int J Antimicrob Agents. 2014 May;43(5):395-402. doi: 10.1016/j.ijantimicag.2013.12.009. Epub 2014 Jan 22. Int J Antimicrob Agents. 2014. PMID: 24503221 Review.
-
Candida and candidaemia. Susceptibility and epidemiology.Dan Med J. 2013 Nov;60(11):B4698. Dan Med J. 2013. PMID: 24192246 Review.
Cited by
-
Virulence and biofilms as promising targets in developing antipathogenic drugs against candidiasis.Future Sci OA. 2020 Feb 3;6(2):FSO440. doi: 10.2144/fsoa-2019-0027. Future Sci OA. 2020. PMID: 32025329 Free PMC article. Review.
-
Is the C-Terminal Domain an Effective and Selective Target for the Design of Hsp90 Inhibitors against Candida Yeast?Microorganisms. 2023 Nov 22;11(12):2837. doi: 10.3390/microorganisms11122837. Microorganisms. 2023. PMID: 38137982 Free PMC article.
-
Screening Repurposing Libraries for Identification of Drugs with Novel Antifungal Activity.Antimicrob Agents Chemother. 2020 Aug 20;64(9):e00924-20. doi: 10.1128/AAC.00924-20. Print 2020 Aug 20. Antimicrob Agents Chemother. 2020. PMID: 32660991 Free PMC article. Review.
-
Postbiotics in the Bakery Products: Applications and Nutritional Values.Probiotics Antimicrob Proteins. 2025 Feb;17(1):292-314. doi: 10.1007/s12602-024-10327-y. Epub 2024 Jul 27. Probiotics Antimicrob Proteins. 2025. PMID: 39066881 Review.
-
Augmenting Azoles with Drug Synergy to Expand the Antifungal Toolbox.Pharmaceuticals (Basel). 2022 Apr 14;15(4):482. doi: 10.3390/ph15040482. Pharmaceuticals (Basel). 2022. PMID: 35455479 Free PMC article. Review.
References
-
- Gudlaugsson O, Gillespie S, Lee K, Vande Berg J, Hu J, Messer S, Herwaldt L, Pfaller M, Diekema D. Attributable mortality of nosocomial candidemia, revisited. Clin Infect Dis. 2003;37:1172–1177. - PubMed
-
- Horn DL, Neofytos D, Anaissie EJ, Fishman JA, Steinbach WJ, Olyaei AJ, Marr KA, Pfaller MA, Chang CH, Webster KM. Epidemiology and outcomes of candidemia in 2019 patients: data from the prospective antifungal the rapy alliance registry. Clin Infect Dis. 2009;48:1695–1703. - PubMed
-
- Wisplinghoff H, Bischoff T, Tallent SM, Seifert H, Wenzel RP, Edmond MB. Nosocomial bloodstream infections in US hospitals: analysis of 24,179 cases from a prospective nationwide surveillance study. Clin Infect Dis. 2004;39:309–317. - PubMed
-
- Morace G, Borghi E. Fungal infections in ICU patients: epidemiology and the role of diagnostics. Minerva Anestesiol. 2010;76:950–956. - PubMed
-
- Lehrnbecher T, Frank C, Engels K, Kriener S, Groll AH, Schwabe D. Trends in the postmortem epidemiology of invasive fungal infections at a university hospital. J Infect. 2010;61:259–265. - PubMed
LinkOut - more resources
Full Text Sources
