Endotoxin-Induced Tryptophan Degradation along the Kynurenine Pathway: The Role of Indolamine 2,3-Dioxygenase and Aryl Hydrocarbon Receptor-Mediated Immunosuppressive Effects in Endotoxin Tolerance and Cancer and Its Implications for Immunoparalysis
- PMID: 26881062
- PMCID: PMC4736209
- DOI: 10.1155/2015/973548
Endotoxin-Induced Tryptophan Degradation along the Kynurenine Pathway: The Role of Indolamine 2,3-Dioxygenase and Aryl Hydrocarbon Receptor-Mediated Immunosuppressive Effects in Endotoxin Tolerance and Cancer and Its Implications for Immunoparalysis
Abstract
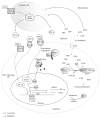
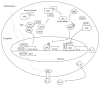
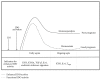
The degradation of tryptophan (TRP) along the kynurenine pathway plays a crucial role as a neuro- and immunomodulatory mechanism in response to inflammatory stimuli, such as lipopolysaccharides (LPS). In endotoxemia or sepsis, an enhanced activation of the rate-limiting enzyme indoleamine 2,3-dioxygenase (IDO) is associated with a higher mortality risk. It is assumed that IDO induced immunosuppressive effects provoke the development of a protracted compensatory hypoinflammatory phase up to a complete paralysis of the immune system, which is characterized by an endotoxin tolerance. However, the role of IDO activation in the development of life-threatening immunoparalysis is still poorly understood. Recent reports described the impact of inflammatory IDO activation and aryl hydrocarbon receptor- (AhR-) mediated pathways on the development of LPS tolerance and immune escape of cancer cells. These immunosuppressive mechanisms offer new insights for a better understanding of the development of cellular dysfunctions in immunoparalysis. This review provides a comprehensive update of significant biological functions of TRP metabolites along the kynurenine pathway and the complex regulation of LPS-induced IDO activation. In addition, the review focuses on the role of IDO-AhR-mediated immunosuppressive pathways in endotoxin tolerance and carcinogenesis revealing the significance of enhanced IDO activity for the establishment of life-threatening immunoparalysis in sepsis.
Figures

Similar articles
-
Indoleamine 2,3-Dioxygenase Activity-Induced Acceleration of Tumor Growth, and Protein Kinases-Related Novel Therapeutics Regimens.Adv Exp Med Biol. 2021;1275:339-356. doi: 10.1007/978-3-030-49844-3_13. Adv Exp Med Biol. 2021. PMID: 33539022
-
Interplay between Estrogen, Kynurenine, and AHR Pathways: An immunosuppressive axis with therapeutic potential for breast cancer treatment.Biochem Pharmacol. 2023 Nov;217:115804. doi: 10.1016/j.bcp.2023.115804. Epub 2023 Sep 15. Biochem Pharmacol. 2023. PMID: 37716620 Review.
-
Associations of microbial and indoleamine-2,3-dioxygenase-derived tryptophan metabolites with immune activation in healthy adults.Front Immunol. 2022 Sep 29;13:917966. doi: 10.3389/fimmu.2022.917966. eCollection 2022. Front Immunol. 2022. PMID: 36248784 Free PMC article.
-
Pharmacologic Induction of Endotoxin Tolerance in Dendritic Cells by L-Kynurenine.Front Immunol. 2020 Mar 11;11:292. doi: 10.3389/fimmu.2020.00292. eCollection 2020. Front Immunol. 2020. PMID: 32226425 Free PMC article.
-
Aryl hydrocarbon receptor and kynurenine: recent advances in autoimmune disease research.Front Immunol. 2014 Oct 29;5:551. doi: 10.3389/fimmu.2014.00551. eCollection 2014. Front Immunol. 2014. PMID: 25400638 Free PMC article. Review.
Cited by
-
Redox regulation of the immune response.Cell Mol Immunol. 2022 Oct;19(10):1079-1101. doi: 10.1038/s41423-022-00902-0. Epub 2022 Sep 2. Cell Mol Immunol. 2022. PMID: 36056148 Free PMC article. Review.
-
Limitations and Off-Target Effects of Tryptophan-Related IDO Inhibitors in Cancer Treatment.Front Immunol. 2019 Jul 30;10:1801. doi: 10.3389/fimmu.2019.01801. eCollection 2019. Front Immunol. 2019. PMID: 31417567 Free PMC article. Review.
-
Urinary metabolomics reveals kynurenine pathway perturbation in newborns with transposition of great arteries after surgical repair.Metabolomics. 2019 Oct 28;15(11):145. doi: 10.1007/s11306-019-1605-3. Metabolomics. 2019. PMID: 31659512 Free PMC article.
-
Patients with hypothermic sepsis have a unique gene expression profile compared to patients with fever and sepsis.J Cell Mol Med. 2022 Apr;26(7):1896-1904. doi: 10.1111/jcmm.17156. Epub 2022 Mar 1. J Cell Mol Med. 2022. PMID: 35934940 Free PMC article.
-
Reverse Iontophoretic Extraction of Skin Cancer-Related Biomarkers.Pharmaceutics. 2021 Dec 29;14(1):79. doi: 10.3390/pharmaceutics14010079. Pharmaceutics. 2021. PMID: 35056976 Free PMC article.
References
-
- Huttunen R., Syrjänen J., Aittoniemi J., et al. High activity of indoleamine 2, 3 dioxygenase enzyme predicts disease severity and case fatality in bacteremic patients. Shock. 2010;33(2):149–154. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

